This application note describes the cultivation of Chinese hamster ovary (CHO) suspension cells in the Finesse SmartGlass vessel bioreactor with a maximum working volume of 2.0 L. Using chemically defined minimal media, cell densities of up to 7.44 10
6
cells/mL were achieved. Recombinant secreted embryonic alkaline phosphatase (SEAP) expression was induced by medium exchange and temperature shift. Maximum SEAP activities of up to 63 U/mL were reached. A novel stirred glass bioreactor suitable for cell culture applications at benchtop scale was designed by Finesse Solutions, Inc. With a maximum working volume of 2.0 L, this bioreactor is controlled by the Finesse G3Lab controller.
Introduction
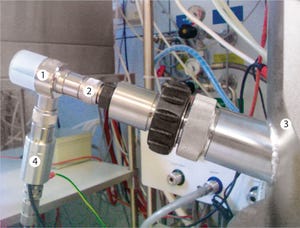
The present study focuses on the cultivation of CHO suspension cells in fed-batch mode using chemically defined minimal culture media and SEAP expression, which is induced by medium exchange and temperature shift. The bioreactor was agitated by a combination of a modified Rushton turbine and a three-bladed segment ...

 +1
+1Successful cell culture on a large scale requires close control of the culture environment in terms of temperature, pH, removal of waste products, and addition of fresh nutrients. To produce high yields of a desired protein, precise control over such parameters at every step in the process is required. Additionally, FDA regulations require various steps to be taken to control bioburden and maintain sterility. These controls and regulations in cell culture environments have led to an increasing move toward single-use systems with the goal of achieving reduced cleaning requirements, high levels of sterility, and zero batch-to-batch contamination (
1
).
Single-Use Solutions
FIGURE 1: DHX cooling efficiences measured by temperature vs. time
Because of contamination concerns, single-use bag systems have gained widespread acceptance and are commonplace in biopharmaceutical manufacturing. More recently, single-use bioreactors for cell culture, single-use tangential-flow filtration (TFF) systems for harvest, and ...
Ladies and gentlemen, welcome aboard ALLpaQ AIR. We have some preflight information about how this bioprocess container optimizes the air freight of media held under temperature-controlled conditions in an environmental chamber.
Before lift-off, please place a single-use bag inside a bioprocess container, fill your media into the bag, and to maintain product temperatures load the bioprocess container inside an Envirotainer RAP e2 container.
Now take count of how many passengers fit inside. One? Two? Three? Four? Owing to the dimensions of standard bioprocess containers, healthcare companies have previously been able to ship only two units per Envirotainer RAP e2 container.
Does this match your calculations? If you could fit only two bioprocess units inside this environmental container, then surely you’d like to fit four? After all, the more media your company ships per flight, the more you save it’s not a case of less is more, but rather more is less.
AIR’s modified footprint doubles the payload, allowing...
Cell culture is widely used for stable expression of monoclonal antibodies with posttranslational modifications. Despite having a stable cell line, a protein product is still in danger of improper modifications from chemical interactions with media. For example, the primary amino groups on a protein are susceptible to reactions with reducing sugar molecules. In the case of a reaction with glucose, this is called
glycation
. The relationship between glucose concentration and glycation is explored here.
Methods:
FIGURE 1: Glucose measurements from three fed-batch runs. Run 1 (square) and run 2 (triangle) were fed once daily. Run 3 (circle) was fed continuously
Chinese hamster ovary (CHO) cells were grown in fed-batch mode in a 2-L glass bioreactor. Offline glucose measurements were obtained using a YSI 7100 system with glucose membrane and two-point calibration. Online measurements were obtained with Biosenz analyzer, with glucose sensor and automated four-point calibration. Glycation of proteins was determ...
Frozen storage is commonly achieved using single-use containers that enable manufacturing process flexibility and long-term product stability as well as minimize logistics challenges. Single-use containers also are available for storing and transporting frozen products although some require a significant investment, and others don’t offer necessary support and protection.
Charter Medical recently developed a single-use frozen storage and transport shipping solution designed to complement single-use Freeze-Pak™ STS (FP-STS) biocontainers, which are engineered specifically for frozen storage applications. The bags and shippers are tested for frozen storage and transport to -80°C. FP-STS single-use biocontainers and shippers have been designed to provide a simple solution while delivering the flexibility and durability required for frozen storage and transport.
The purpose of the study presented here was to test the new Freeze-Pak™ STS single-use secondary storage and shipping containers (Charter Medical, Lt...
Through the combined strengths of the New Brunswick™ and DASGIP® product portfolios, Eppendorf now has the product portfolio and expertise to provide an outstanding range of premium equipment and single-use solutions to meet all needs.
A New Scale of Bioprocessing
From the parallel mini bioreactor system DASbox for early stage bioprocess development to the benchtop and parallel bioreactor systems for laboratory scale and the sterilize-in-place solutions for production, Eppendorf offers industry and research users its extensive bioprocess solutions from a single source while meeting the highest quality demands.
Eppendorf Bioprocess Software: Much More Than Just Bioprocess Control
Eppendorf offers BioCommand® and DASGIP Control supervisory control and data acquisition (SCADA) software packages for advanced bioprocess control. next-generation bioprocess management is provided by the comprehensive DASware software suite.
The BioCommand software for New Brunswick line controllers enhances your ability to monit...
With the largest network of harmonized biopharmaceutical good manufacturing practice (GMP) product testing labs worldwide, Eurofins BioPharma Product Testing provides comprehensive laboratory services for the world’s largest pharmaceutical, biopharmaceutical, and medical device companies. We successfully enable companies to advance candidates from development through commercialization while ensuring regulatory compliance, cost effectiveness, and achievement of timelines.
The Most Comprehensive Range of Large- and Small-Molecule Testing Services Available Worldwide
Choose from a wide range of testing services that support all functional areas of biopharmaceutical drug development and manufacturing, including method development, microbiology, process validation, and quality control. Our service offerings are fully comprehensive and include testing of drug substances, final products, intermediates, and starting materials for both small- and large-molecule drug products, including
Global Facilities
Work with ...
Danish-based FeF Chemicals is a preferred specialist supplier of premium quality ingredients for the biopharmaceutical and pharmaceutical industries across two unique product areas: insulin human AF for cell culture media and current good manufacturing practice (CGMP)-manufactured quaternary ammonium compounds (QUATS). And although high quality, innovation, flexibility, and security of supply are among its market-leading features, end-user patients are the motivators. FeF Chemicals supplies insulin human AF (for use in cell culture media) from Novo Nordisk, the largest insulin manufacturer worldwide.
Strong Historic Foundation:
The company was first established in 1949. It was acquired by Novo Nordisk in 1986 and has been part of the pharmaceutical group since then. Novo Nordisk is a renowned global leader with over 90 years of innovation and leadership in diabetes care. The group’s core values are centered on improving the lives of patients. It also strives for excellence, is accountable to customers and...
As major economies in the Asia–Pacific region continue to increase focus on healthcare modernization, new opportunities for both global and local biopharmaceutical companies are being created. Such opportunities are driving demand for local sourcing of raw materials to ensure security of supply.
Modernization of Healthcare Drive Opportunities for Biopharmaceutical Firms
Recent modernization of healthcare by governments has seen China pledge 124 billion to improve its healthcare system to provide insurance to 90% of its residents (
1
). South Korea introduced a biosimilar regulatory pathway consistent with EMA guidelines (
2
) and launched its Bio-Vision 2016 initiative to invest 14.3 billion in 10 years (
3
). India issued its guidelines on biosimilars (
4
) to provide a pathway for approval. And Singapore continues to invest in R&D with its Agency for Science, Technology and Research by signing a five-year strategic agreement with GSK to develop evidence-based formulations targeted specifically for emerg...
To achieve good growth of microorganisms and high yields in protein expression, the following factors in fermentation are important: medium, gas
Fermentors for research and development have to meet very high standards for flexibility so that they can be easily adapted to the requirements of a wide range of microorganisms and process conditions. For that purpose, modular fermentation systems were developed, consisting of single modules such as drive, fermentation vessel, analytical instrumentation, stirrer, foam separator, ventilation, and so on. This allows for individual assembly and upgrades.
metabolism, temperature, pH, and pressure.
The concentration of dissolved oxygen (DO) in medium (proportional to the pO
2
value, which is the partial oxygen pressure that is determined as DO) is an important parameter of gas metabolism. The Hamilton VisiFerm optical DO sensor was tested for four months monitoring this parameter.
The Measurement SiteThe rate of the stirring speed and air flow starts with a defau...
Media and buffer preparation is a key part of the biopharmaceutical manufacturing process. Although this step doesn’t have to be carried out in sterile conditions, improving the powder transfer process makes preparation cleaner, safer, and more efficient while protecting personnel and cutting time and costs.
The Issues of Powder Handling
Powder ingredients for media and buffers have historically been transferred using scoops and weighed and mixed in buckets or open top bags. Many companies are starting to move to single-use systems, but the transfer of dry ingredients still results in fine airborne particles that can cause employee health issues and a slight risk of ignition.
Airborne particles eventually settle onto surfaces, so cleaning between batches must be performed to prevent contamination. This task lengthens the time needed for changeover and increases labor costs.
Process waste also results from using disposable bags that are difficult to fill quickly and accurately. And systems can trap powders...
In the field of research and development into the production of bioactive compounds, there is incredible demand for small-scale cultivation systems that operate in the liter range (1, 2). Single-use bag bioreactors can close the gap between cell and media screening devices (at scales of up to several hundreds of milliliters) and pilot bioreactor systems at scales larger than several hundreds of liters (3). The incubator shaker Multitron Cell with ShakerBag Option, in particular, represents an easy and economical solution for increasing cultivation volumes beyond classical shake-flask scales without the need to purchase a bioreactor system. Besides the general advantages that arise from the single-use design of these bag bioreactors (4), they are also characterized by reduced foam formation and flotation due to the surface aeration (4, 5) that plays an important role in plant cell suspension-based processes.
Identifying engineering parameters such as mixing time, oxygen mass transfer, and power input is im...

 +2
+2Growing adoption of single-use bags for the production of biopharmaceutical drugs raises new challenges, including consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, Sartorius Stedim Biotech’s polymer scientists and biologists have followed a quality by design (QbD) program for the development of a completely new polyethylene film S80 thus achieving consistent performance of the new Flexsafe bags for all bioprocess steps and applications. The partnerships and agreements with our suppliers, the complete understanding and control of our manufacturing process from the resin, and the film extrusion to the final sterile bioprocessing bag are the prerequisites to ensure reliability of our supply chain. This assurance of supply relies on a long-term contract with our film supplier. In addition, the establishment of resin specification instead of general trademarks provides robust change contro...