Single-use (disposable) systems are being considered and introduced into many biopharmaceutical processes because manufacturers have identified significant benefits they offer over traditional reusable systems. These benefits are often more evident when a new process and product are being developed. Lower capital expenditures, shorter development times for new facilities, and reduced validation costs are some of the reasons single-use technology may be selected. Here, a contract manufacturer’s case study is described in which an existing stainless steel system was completely replaced with a ∼90% disposable manufacturing train. The specific benefits gained by this change were improved product quality, substantially reduced processing times, and lower operating costs.
Changing an existing reusable system to a disposable system may not always seem attractive. In a recent survey of manufacturers, 65% of respondents stated that investment in existing equipment restricted their implementation of disposables, as shown in Table 1 (1). However, the data also showed that contract manufacturing organizations (CMOs) were less restricted by legacy systems, indicating that other factors can contribute significantly to a justification for disposables by CMOs (Table 2).
Table 1: Reasons given for restricting disposables use, comparing 2006 and 2007 results (1)
Table 1: Re asons given for restricting disposables use, comparing 2006 and 2007 results (1) ()
Table 2: Reasons given for restricting disposables use, comparing answers from biomanufacturers and contract manufacturers (1)
Table 2: Re asons given for restricting disposables use, comparing answers from biomanufacturers and contract manufacturers (1) ()
In many processes, assessment of the possible replacement of existing stainless steel systems with disposable components may be a worthwhile exercise because it can often realize specific and sometimes unexpected benefits. For example, the survey listed some critical reasons identified by manufacturers for increasing their use of disposables. Table 3 shows the five most important reasons given. Such an assessment may show advantages in converting either an entire system or just a specific part of it to create a hybrid system, which may be ultimately convertible to a fully disposable process at a later date.
Table 3: Cr itical reasons for increasing disposables use (1) ()
Table 3: Critical reasons for increasing disposables use (1)
Table 3: Cr itical reasons for increasing disposables use (1) ()
The Product and Manufacturing Process
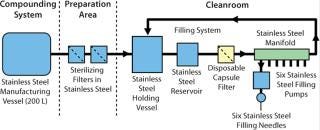
The product in our case study was at the phase 2 development stage, with batches being manufactured using a typical stainless steel manufacturing train (Figure 1). A decision was then made by the company to outsource and transfer the process to a contract manufacturer, Patheon UK, with an initial requirement to produce sufficient quantities for clinical trials. The longer-term requirement was to scale up the process to full production status for product launch.
Figure 1: ()
Initial results from small-scale batches manufactured by Patheon revealed concerning levels of related impurities at release and over the shelf-life of the product. On investigation, the cause of this product degradation was identified as a formulation sensitivity to stainless steel and oxygen. The sensitivity to stainless steel was of special significance because it gave Patheon the opportunity to evaluate disposable components, thereby eliminating stainless steel entirely from the manufacturing process. The company had already been researching and introducing single-use technologies such as bioprocess containers and filter capsules into its processes and had completed several projects incorporating fully disposable manufacturing trains. This experience would be of value in resolving the product impurity issue as well as introducing other possible benefits.
Project Approach
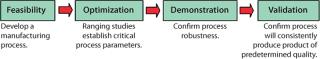
Qualification and validation of the disposable system were planned together in four stages (Figure 2). Patheon recognized from previous experience that a well-prepared project plan was vital to successful development of the single-use system. The plan included allocation of resources, formation of an ideal project team, defining the schedule, and listing the requirements for documentation and validation. Most important, effective preparation and implementation of the plan required resources from both the CMO and its client. There was a need to understand how the system would be used regarding storage locations, materials flow, requested support hardware, and methods of connection and disconnection. It was also essential to develop a testing strategy, depending on the applications. A user requirements specification (URS) formed the basis for identifying the various components required at each step of the process and involved close collaboration with suppliers of equipment and materials.
Figure 2: ()
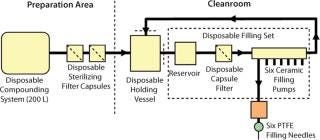
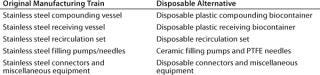
The basic design of the existing manufacturing train can be divided into five major stages, and each could be assessed separately for suitable alternative disposable components. Table 4 compares the original and proposed alternative systems. Figure 3 provides a schematic representation of the disposable manufacturing system.
Figure 3: ()
Table 4: Comparing original and disposable manufacturing systems
Table 4: Co mparing original and disposable manufacturing systems ()
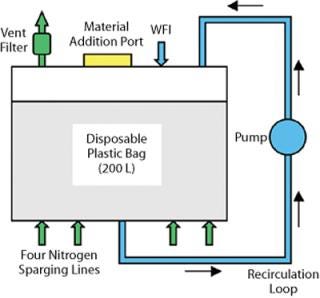
Compounding Vessel: Development of a suitable disposable compounding vessel involved strong collaboration with a bioprocessing bag manufacturer. Two critical requirements had to be satisfied in the design: The system had to be capable of effective and rapid dissolution of materials, and fluid oxygen levels had to be maintained at <0.5 ppm throughout the process. A three-dimensional 200-L compounding biocontainer system was successfully developed that excluded stainless steel from all liquid contact surfaces and fully satisfied the requirements of the URS. The system contained several key features, including a pumped recirculation loop to provide effective mixing, four inlet ports for nitrogen sparging to minimize oxygen content, a vent port and filter for nitrogen release, and inlet ports for material addition and water for injection. Figure 4 shows its design.
Figure 4: ()
Recirculation/Filling Sets: Development of a suitable disposable recirculation set involved strong collaboration with Pall Life Sciences’ biopharmaceuticals group to produce a fully disposable configuration of a filling manifold, reservoir, and feed and return lines. Ceramic filling pumps from IMA replaced stainless steel rotary valve filling pumps, and polytetrafluoroethylene (PTFE) filling needles were sourced from Bosch.
The materials of construction chosen were organic polymers to eliminate stainless steel, except for the pump and filling needles that were ceramic and PTFE. When compared with the original stainless steel manufacturing train, higher levels of extractables would be expected from this high organic polymer content. However, the component suppliers were able to provide a substantial amount of standard validation data including biological safety per USP Class VI for plastics; general compatibility and biocompatibility data; and extractables data on nonvolatile, semivolatile, and volatile residues using a model solvent approach with water for injection (WFI), base, acid, and a worst-case organic solvent. The testing regime also enabled analysis for total organic carbon (TOC), pH shift, and conductivity using WFI as a solvent. This considerable amount of validation data was then used as part of a risk assessment for leachables and compatibility. The standard extractables data from the supplier were used in the validation documentation and supported by product-specific compatibility and leachables data generated by Patheon.

Figure 1:
Performance of the Disposable System
The most critical requirement in the URS was improvement of product quality. This was achieved in the disposable system by eliminating stainless steel (the main cause of the product-related impurities) and maintaining oxygen levels at <0.5 ppm. With that main objective achieved, a detailed analysis of processing times for the disposable system was performed and compared with data from the original stainless manufacturing train (Table 5). Those data reveal a dramatic time savings of over tenfold, reducing the total processing time from 19 hours to 1.5 hours and substantially reducing operating costs as well as providing greater manufacturing capacity each day. Additional cost savings arising directly from use of a disposable system would be expected (e.g., labor, WFI, cleaning chemicals, and equipment maintenance). For a new process rather than replacement of an existing process, additional factors such as purchase of capital equipment, development time, and (most important) validation costs would yield additional cost benefits.
Table 5: Comparing disposable and nondisposable systems
Table 5: Co mparing disposable and nondisposable systems ()
Establishment of Dedicated Disposable Filling Lines
As a consequence of establishing a disposable manufacturing train for the case study detailed in this article, Patheon identified at first hand the advantages of using disposables. Taking this into account, as well as the nature of Patheon’s business of contract drug product manufacturing, Patheon is now establishing dedicated disposable development and commercial manufacturing lines.
Further Development and Scale-Up: Development of a disposable filling system for Patheon’s IMA–Flexicon filling line involved collaboration with Pall Life Sciences, a manufacturer of disposable components and systems. The main requirements were a bioburden reduction prefilter, a choice of sterilizing grade filters to accommodate different batch sizes, integrity testing of the sterilizing filter on line, a selection of receiving vessels ranging from two to 200 L, a second sterilizing grade filter, a filling manifold with four inlet/outlet lines at a range of tubing diameters, and filling needles for 0.2–22 mL volumes, all supporting typical capacity of ≤10,000 units of product per batch. A disposable filling system (Figure 5) was designed and developed based on these requirements to provide product for clinical trials and small-scale manufacture.
Figure 5: ()
Future Scale-up and Improvements to Process: The disposable system described here can produce typically ≤10,000 units per batch with 0.25–25 mL fill volumes. An ongoing project is now addressing scale-up of this system to accommodate larger batches incorporating a peristaltic filling system sufficient to produce >10,000 units for phase 3 clinical materials and larger commercial batches. These developments will ensure that Patheon’s facility in Swindon, UK, will have dedicated aseptic vial filling capabilities for filling small-batch sizes up to large commercial-scale batches using fully disposable processes. The facility will enable rapid product changeover, giving greater production flexibility and capacity.
Solving Multiple Problems
This case study shows how introduction of a single-use system resolved a very specific product quality problem and at the same time provided cost benefits by replacing a functional stainless steel system. In addition, some developments were transferable to other processes.
For contract manufacturers such as Patheon, disposables can offer particular benefits associated with the need for multiproduct, versatile production facilities to meet their clients’ requirements and provide a competitive business approach. However, many of the benefits realized in this project are also applicable to companies manufacturing products in their own facilities, whether replacing an existing nondisposable system or developing a brand new process. In-depth analyses and comparative data on the wider aspects of single-use systems in a process facility enable manufacturers to assess more accurately the potential benefits of disposable components in a specific process system (2, 3).
The rapid developments in single-use technology over recent years have significantly widened its spectrum of applications. Introduction of these systems into biopharmaceutical manufacturing should accelerate as other new technologies become available.

Figure 2:
REFERENCES
1.) Langer, E. 2008.Fifth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates Inc., Rockville.
2.) Sinclair, A, and M. Monge. 2004. Concept Facility Based on Single-Use Systems, Part 1. BioProcess Int. 2:S26-S31.
3.) Sinclair, A, and M. Monge. 2005. Concept Facility Based on Single-Use Systems, Part 2. BioProcess Int. 3:51-55.