If current efforts to eradicate polioviruses worldwide are successful, then the oral poliovirus vaccine (OPV) currently used for routine immunization in low- and middle-income countries (LMICs) will be replaced by inactivated poliovirus vaccine (IPV). IPV will become the only option for such countries if they want to continue to vaccinate against polio (1). Because IPV is currently considered to be too expensive for use in LMICs, strategies are being undertaken to make IPV more affordable (2). Some experts estimate that in 5–10 years there will be a lack of IPV manufacturing capacity worldwide (3). That will most affect LMICs, which depend on external vaccine supplies. So expansion of existing facilities (or building new ones) will be key to meet future demand. Here we examine the use of alternative technologies such as modular manufacturing facilities and single-use equipment as means to improve IPV affordability and capacity.
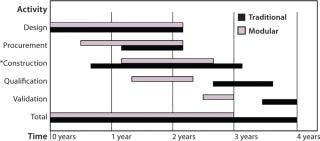
Figure 1:
Main Drivers for Implementation of Modular Facilities and Disposables in Vaccine Production: One major argument for modular facilities is their time savings over a conventional construction approach (4). Even though the initial cost for using modular facilities exceeds that of conventional methods, an assurance of predictable outcome and time savings provides earlier return on investment for clear cost savings. Time is saved by conducting activities in parallel, which allows for significant compression of tasks (Figure 1). With all construction undertaken in a controlled environment, results are consistent and quality is enhanced. Equipment installed within each module (at its final operating location) will require no reassembly or extensive retesting. The building and fit-out of modules to good manufacturing practice (GMP) specifications in an external environment provides further benefit when applied to LMICs because technical skills required to complete and validate a facility to suitable specifications would not be required within the country itself.
Figure 1: ()
Single-use technologies typically allow for a simpler facility design than their stainless-steel counterparts because complex clean-in-place (CIP) and steam-in-place (SIP) systems are no longer required to support the equipment between operations. So disposables facilitate faster design, construction, and commissioning of facilities as well as offering manufacturing flexibility through the reconfiguration of manufacturing suites. A smaller, less complicated facility can be built, commissioned, qualified, and validated in 12–18 months rather than four years (5).
The scale of viral vaccine production is generally smaller than that of many other processes (ranging 500–2,000 L), so known limitations of scale involved with single-use technologies (max 2,000 L) can be advantageous for this application. Compared with traditional approaches, single-use technology offers many benefits for vaccine manufacturing (6, 7):
Fast turnover maximizes facility output (no CIP or SIP operations).
Reduction of capital investments is linked to reduction and simplification of facility design and to lowered equipment investment.
Viral production must be handled in a biosafety containment level 3 environment. Single-use technology simplifies production areas because of smaller equipment footprints, lower ceiling heights, and elimination of water-for-injection and steam utilities.
Our aim is to evaluate the potential of using these alternative technologies — modular facilities and single-use technology — for affordable and sustainable IPV production in LMICs.
Approach and Methodology
We evaluated the affordability of IPV based on a cost-of-goods-sold (COGS) analysis and the resulting manufacturing cost per dose. We determined the economic sustainability of a facility by simulating cash-flow, net present value (NPV), and return on investment (ROI). Using those criteria, we compared the feasibility of three scenarios:
Traditional (baseline) — manufacturing IPV in a traditional facility using standard stainless steel equipment
Hybrid — manufacturing IPV in a modular facility using a mixture of standard stainless-steel equipment (filtration and chromatography columns) with some single-use technology (bioreactor, product hold, and buffer/media preparation)
Disposable — manufacturing IPV in a modular facility using only single-use technology throughout.
We used the commercially available Biosolve process software from Biopharm Services to undertake our COGS analysis, to simulate the project’s cash flow, and to estimate NPV and ROI.
IPV Process and Assumptions
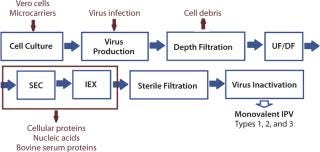
The IPV production process details and sequence of operations used in BioSolve process software for analysis of all scenarios are based on a process (Figure 2) developed by Van Wezel in 1989 (8). It is meant for production of any type of monovalent bulk supply of IPV (type 1, type 2 or type 3). Once manufactured, those monovalent bulk types are combined to make a trivalent bulk vaccine.
Figure 2: ()
Assumptions: We considered all facilities to be “new builds” and used the same scale of operation throughout all facilities/scenarios. Single-use technology was assumed to achieve similar performance levels to their stainless steel counterparts. Modular facilities have the same cost of construction as traditional facilities.
Results
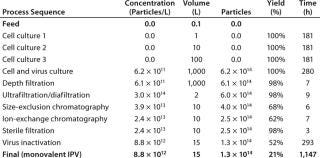
IPV Process: The IPV manufacturing process used in BioSolve for analysis of all scenarios is based on a 1,000-L scale process developed at the Netherlands National Institute for Public Health and the Environment (8). The Biosolve program provided IPV manufacturing process d
etails (Table 1) based on a 1,000-L virus culture with an expression level of 6.2 × 1011 virus particles per liter of culture and an 80% facility capacity. That gave an overall yield of 21% in a process total duration of 1,147 hours (48 days).
Table 1:
Table 1:� 194; ()
Baseline Scenario — COGS Analysis: The traditional facility (with only stainless steel equipment used throughout) represented a baseline scenario for comparison with alternative scenarios using modular facilities and single-use technology. Table 2 summarizes yearly process outputs and resulting cost/dose of monovalent IPV for this baseline. These results were successfully validated against a reported throughput of ∼106 doses per run from a 1,000-L virus culture and an estimated production cost for monovalent IPV of US$1.50/dose (8). The production process, number of batches per year, and throughput (number of doses per year) defined here were the same for all scenarios evaluated.
Table 2:
Table 2:� 194; ()
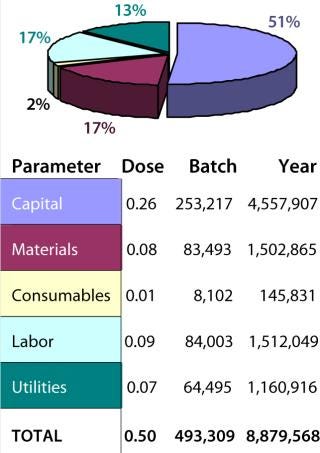
Figure 3 summarizes the COGS breakdown of the baseline obtained from Biosolve analysis. The cost/dose of IPV is dominated by capital costs, which represent 51% of all costs. That is the result of costly traditional facilities with stainless-steel equipment.
Figure 3: ()
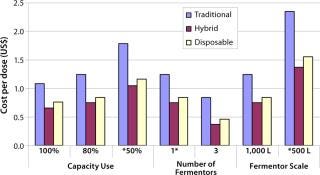
Baseline Scenario — Sensitivity Analysis: We also analyzed the impact of operational variables such as capacity use, the number of fermentor vessels, and the operational scale on IPV production cost/dose for each scenario (Figure 4). Our sensitivity analysis results suggest that increasing the throughput of a facility by either running it at maximum capacity (100%), increasing the operation scale (to 1,000 L), or increasing the number of virus-production vessels (3 × 1,000 L) would provide significant cost savings and result in lower manufacturing cost/dose of IPV. The lowest cost/dose was achieved using three fermentor vessels. Vaccine manufacturing involves economies of scale. Increasing the number of fermentor vessels could be gradually phased into a facility by building add-on modular upstream suites as required. The increase in capacity would not be easily achieved in a traditional facility using stainless steel.
Figure 4: ()
Assumptions for Cash-Flow, NPV, and ROI Analyses
Price per dose: baseline cost per dose (US$0.50)
10-year project duration beginning in 2013
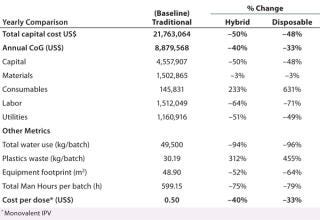
Single-Use Technology — COGs Analysis: We assessed two alternative scenarios using single-use technology for IPV production and compared them with the baseline: a hybrid facility using a combination of stainless-steel equipment and single-use technology and a fully disposable facility using only single-use technology. Table 3 summarizes results of the COGS analysis with a percentage of change between each scenario and the baseline.
Table 3:
Table 3:� 194; ()
Using single-use technology (in either a hybrid or fully disposable facility) provided the following results:
Reduction of capital cost by ≤50% by replacing stainless-steel with single-use equipment (capital cost having been established as the main driver for increasing the affordability of IPV)
Reduction of equipment footprint (by 52–64%), which contributes to a smaller facility and therefore lower capital investment
Increase in consumables and waste generation (plastics) by 631% and 455%, respectively — requiring appropriate incineration facilities to treat plastic waste
Reduction of labor cost by ≤71% (total work hours reduced by ≤79%) and utilities cost by ≤51% (water consumption reduced by ≤96%) with fewer cleaning requirements of single-use technology — a significant advantage in LMICs, where clean water, waste cleaning facilities, and qualified labor are in their infancies and frequently unreliable
Reduction of overall annual COGS by ≤40% and, as a result, also a reduction in the manufacturing cost/ dose of IPV.
The hybrid facility, however, presented greater cost savings than did the fully single-use facility and provided the lowest cost
/dose of IPV. Increased COGS from the that facility were mainly due to the additional costs associated with the ultrafiltration/diafiltration (UF/DF) step and prepacked columns. However, that facility presented greater advantages than the hybrid facility because it lowered water consumption, reduced labor requirements (work hours) due to fast set-up, and decreased space requirements for the equipment, storage, and cleaning operations (for a smaller overall facility footprint).
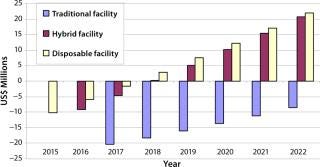
Modular Facility — Cash-Flow Analysis, NPV, and ROI: We undertook a cash-flow analysis of these scenarios to take into account the use of modular facilities (Figure 6). It was previously assumed that modular facilities would have the same cost of construction as traditional facilities, so only through this analysis could we examine their impact on investment.
Figure 5: ()
Previously it has been shown that facilities based on single-use technology can reduce the manufacturing cost per dose of vaccine when compared with wholly stainless steel facilities. We’ve shown that using modular facilities in conjunction with single-use technology makes it an even more attractive option. Combining these technologies provides time savings over a traditional facility construction and consequently offers an earlier return on the initial capital invested. Traditional facilities take longer to build and require higher up-front capital.
Single-use technology alone helps simplify facility design and reduce costs. That reduction arises from the ability to delay expenditures from the start (as with procurement of stainless steel equipment) to production-based spending on consumables (off-the-shelf equipment and ready-to-use components). This shift in cost structure reduces overall costs by moving them to a later point in the life cycle of a facility (9).
Combining modular facilities and single-use technology also allowed for faster builds by reducing floor area required by the facility. And disposable equipment is bought “off-the-shelf.” That lowers design requirements and hence reduces project timelines and leads to an earlier break-even point.
Table 4 shows the NPV, ROI, and break-even points for each scenario we analyzed. Modular facilities with single-use technology gave positive NPV and ROI values as well as lower capital expenditure and a faster break-even point (eight years earlier) than a traditional facility using stainless-steel equipment.
Table 4:
Table 4:� 194; ()
Building Blocks
Economically sustainable access to affordable vaccines is an important component of health care, which in many LMICs continues to be limited. Our study provides a first step toward constructing a road-map that can lead to alternative manufacturing solutions that can be more suitable to the needs and infrastructures available in such countries. We have shown that combining technologies such as modular facilities and single-use technology may provide an answer to self-sustainable and affordable production of IPV to supply LMICs. Using Biosolve software in this way has allowed for cost-based decision making before capital commitment, ensuring that public vaccination strategies represent the best value for money spent.
About the Author
Author Details
Adriana G Lopes is managing director of LLB Global Health Solutions Ltd. in London, UK. Corresponding author Andrew Sinclair is managing director of Biopharm Services, Lancer House, East Street, Chesham HPS 1RD, UK; 44-1494793243; [email protected], www.biopharmservices.com. And Nigel Titchener-Hooker is director of the Advanced Centre of Biochemical Engineering in the Department of Biochemical Engineering at University College London, UK.
REFERENCES
1.) Liu, X. 2003.OPV vs IPV: Past and Future Choice of Vaccine in Global Polio Eradication ProgramThe Partners for Health Reform, ABT Associates Inc., Bethesda.
2.) Hickling, J. 2010.An Economic Analysis of Strategies to Reduce the Cost of Routine IPV Immunization: Improving the Affordability of Inactivated Poliovirus Vaccines (IPV) for Use in Low-and Middle-Income Countries, PATH Publications, Seattle.
3.) Venczel, L. 2009. Global Post-Eradication IPV Supply and Demand Assessment: Integrated Findings, Oliver Wyman, Inc., New York.
4.) Leichter, G, and L. Turstam. 2004.An Alternative Approach to the Modular Design and Construction of Large-Scale Bulk Biopharmaceutical Manufacturing FacilitiesPharmaceut. Eng:8-22.
5.) Sellick, I, and H. Pora. 2011.Single-Use Process Systems, GDS Publishing Ltd, Bristol.
6.) Chaubard, J-F. 2010.Disposable Bioreactors for Viral Vaccine Production: Challenges and OpportunitiesBioPharm Int.
7.) Pora, H. 2006.The Case for Disposable Manufacturing Equipment to Accelerate Vaccine DevelopmentBioPharm Int.
8.) Van Wezel, AL. 1984. Inactivated Poliovirus Vaccine: Current Production Methods and New Developments. Clin. Infect. Dis 6:S335-S340.
9.) Sinclair, A, and M. Monge. 2011. Monoclonal Antibody Manufacturing: Cost Benefits of Hybrid Disposable Systems. BioProcess Int 2011 9:12-17.