At small to medium scales, single-use technology offers significant advantages over traditional reusable (e.g., stainless steel) manufacturing technology with regard to flexibility, cost of goods, implementation timelines, and maintenance. However, process design based on disposables does create new challenges. With traditional fed-batch processes, harvest clarification is usually achieved by centrifugation followed by depth filtration. For processes based entirely on disposables, the disc-stack centrifuge needs to be replaced by filtration alone.
To extend its manufacturing capabilities and capacities, Rentschler decided to build two 1,000-L scale cell culture good manufacturing practice (GMP) lines based exclusively on single-use equipment. During the design phase, the team performed and reported several studies on disposable equipment characterization (1, 2). In this concept study, we evaluated single-use depth filtration systems from different suppliers for potential implementation into a high–cell-density monoclonal antibody platform process at 1,000-L scale (3).
PRODUCT FOCUS: MONOCLONAL ANTIBODIES
PROCESS FOCUS: DOWNSTREAM PROCESSING
WHO SHOULD READ: PROCESS DEVELOPMENT AND MANUFACTURING
KEYWORDS: DEPTH FILTRATION, SINGLE-USE TECHNOLOGY, FED-BATCH
LEVEL: INTERMEDIATE
Experimental Approach
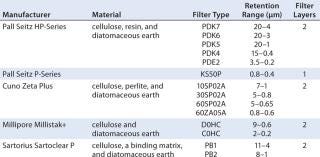
For our first step, we chose fully disposable depth filter systems from Pall, Cuno, Millipore, and Sartorius Stedim Biotech to screen at laboratory scale (Table 1). We evaluated these depth filters (stand-alone or in series) with a total of 117 tests, including eight different 0.2-µm membranes for a final clarification step. For the laboratory-scale tests, we grew high–cell-density fed-batch cultivations of a Chinese hamster ovary (CHO) cell line expression monoclonal antibody (MAb) at 10-L scale in chemically defined media. Harvest occurred after 14 days, with cell viabilities >94%. Table 1: Characteristics of different single-use depth filters tested
Table 1: Characteristics of different single-use depth filters tested ()
Subsequently, we further evaluated the two best-performing single-use depth filtration systems at production scale. Harvest came from the same fed-batch platform process run in three 200-L disposable bioreactors after 14–19 cultivation days. Final viabilities ranged 40–90%. During all filtrations, we applied a constant flow of 100 L • m−1 • h−1. We determined the maximum filter capacities at a pressure of 1 bar. Then we assessed filter performance with regard to maximum capacity, turbidity, and yield.
Results and Discussion
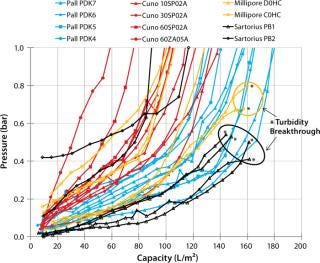
Figure 1 displays the results of our initial screening. At low differential pressures, all the different suppliers’ filters showed exponential curves typical of a filtration process at constant flow. With 130–180 L/m2, the Pall depth filters reached the highest capacities at the defined limit for a differential pressure of 1 bar and gave good filtrate quality throughout — as measured by turbidity values (<10 NTU). The Cuno 10SP02A and 60SP02A depth filters showed good capacities. With 60SP02A, low turbidity values (<10 NTU) were obtained in filtrates as well. Cuno 10SP02A needed to be combined with a second depth filter to prevent turbidities >10 NTU in filtrates.
Figure 1: ()
Low turbidity is required to maintain good performance of the subsequent 0.2-µm filter. We observed turbidity breakthroughs at pressures <1 bar for the Millipore D0CH and Sartorius Stedim PB1 depth filters, with which differential pressures started to plateau at 0.4–0.8 bar in some test runs and failed to reach the defined limit of 1 bar. This type of profile is typically associated with filter breakthrough, which we confirmed by turbidity breakthroughs at the respective pressures.
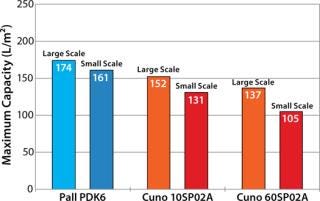
Based on those results, we selected Pall and Cuno depth filters for further evaluation at the 200-L scale and tested them both as stand-alone and in combination followed by a 0.2-µm filter. Figure 2 displays the filter-capacity results, showing that scale-up from laboratory to large-scale yielded reproducible performance with both systems. The Pall PDK6 depth filter system at large scale gave the highest capacity of 174 L/m2, which was 14% and 27% higher than that of the Cuno 10SP02A and Cuno 60SP02A, respectively. Product loss was <10% in all cases.
Figure 2: ()
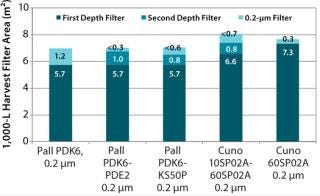
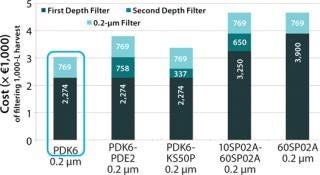
We identified Cuno BioAssure, Pall Supor EKV, Pall Fluorodyne EX EDF, Sartorius Sartopore 2 XLG, and Millipore Express SHC as all suitable sterilizing-grade 0.2-µm filters. And our calculations showed that the overall filter areas needed at 1,000-L scale would be <8 m2 for all combinations tested (Figure 3). Based on a list-price cost comparison for 1,000-L scale then supported selecting the Pall PDK6 filter (Figure 4).
Figure 3: ()
Figure 4: ()
Eliminating Centrifugation
In this concept study, we tested disposable depth-filtration systems for implementation into a MAb platform manufacturing process at 1,000-L scale. Our investigations also showed that clarifying culture harvest with only single-use depth filtration can be an attractive alternative to the traditional fed-batch process design that includes both centrifugation and filtration — both economically and for process intensification.
However, currently available depth-filter modules exhibit significant differences concerning process robustness and capacity. So we identified Pall’s Stax disposable depth filter system equipped with a PDK6 filter in combination with a 0.2-µm downstream filter as our first choice. For our process, this single-use filtration set-up offered high capacities and low product loss as well as simple and convenient handling. Based on these results, Rentschler and Pall will perform further development jointly to optimize both the process and hardware for harvest clarification across a wide range of different cell line and process platforms.
About the Author
Author Details
Corresponding author Antje Pegel ([email protected]) is a process scientist in upstream process development, and Sven Reiser ([email protected]) is a process engineer in upstream production at Rentschler Biotechnologie GmbH Erwin-Rentschler-Straße 21 88471 Laupheim, Germany. Mona Steurenthaler is a single-use systems specialist, and Simone Klein is a senior biopurification specialist at Pall GmbH Life Sciences, Philipp-Reis-Straße 6 63303 Dreieich, Germany; [email protected]
REFERENCES
1.) Dudziak, G. 2010.Full Plastics: Generic Antibody Manufacturing Using Total Disposable TechnologyBioProcess International Conference and Exhibition, Providence, RI, IBC Life Sciences, Westborough.
2.) Laukel, M, P Rogge, and G. Dudziak. 2011. Disposable Downstream Processing for Clinical Manufacturing. Current Capabilities and Limitations. BioProcess Int 9:S14-S21.
3.) Pegel, A. 2011.Evaluation of Disposable Filtration Systems for Harvesting High Cell Density Fed-Batch ProcessesESACT 2011, European Society for Animal Cell Technology, Geneva:15-18.