Mixed-mode chromatography sorbents can save time and money by reducing the number of steps required to purify recombinant proteins. They also have the potential to purify proteins that single-mode sorbents cannot. As the term mixed mode suggests, these sorbents contain ligands that offer multiple modes of interaction. Although mixed-mode sorbents are used extensively in solid-phase extraction for high-pressure liquid chromatography (HPLC) sample preparation — and to a more limited extent in analytical HPLC — these resins are generally unsuitable for protein purification because their pores are too restrictive and they are often too hydrophobic for good protein recovery. Apart from hydroxyapatite, such sorbents are relatively new to the market. Currently they are not widely used in process-scale purification of recombinant proteins. This situation may stem, in part, from the widespread assumption that mixed-mode sorbents are poorly understood, mechanistically, so they would require extensive studies to achieve chromatographic optimization. As described here, that potential impediment does not apply to all mixed-mode sorbents. Based on studies described here, our view is that mixed-mode sorbents generally deserve much more serious consideration in designing process-scale purification schemes than they have received thus far.
PRODUCT FOCUS: PROTEINS
PROCESS FOCUS: DOWNSTREAM PROCESSING (PURIFICATION)
WHO SHOULD READ: PROCESS DEVELOPMENT AND MANUFACTURING
KEYWORDS: CHROMATOGRAPHY, SORBENTS, MIXED MODE, HCIC, HIC, IMAC, IEX, SCALE-UP
LEVEL: INTERMEDIATE

Figure 1:
AVECIA BIOLOGICS LTD (WWW.AVECIA.COM)
Mixed-mode sorbents are innovative for three reasons. First, they can facilitate efficient capture and significant purification in a single chromatographic step because of simultaneous operation of orthogonal modes of interaction. In our study, the mixed-mode sorbent operates by a combination of hydrophobic and electrostatic interaction. Simultaneous operation of multiple interaction modes in a single step may facilitate reduction of a conventional three-step purification scheme to two steps. Second, mixed-mode sorbents provide unique selectivities that are not achievable by single-mode sorbents used sequentially, so they enable some proteins to be purified when single-mode sorbent combinations fail. Third, certain mixed-mode sorbents are capable of reducing endotoxins to clinically acceptable levels.
Case History
Here we describe successful use of a mixed-mode sorbent to purify a recombinant protein from Escherichia coli needed for phase 1 and 2 clinical trials. We developed our purification process after several other methods performed poorly in the capture step. Use of a mixed-mode sorbent produced clinical-grade material after only two chromatographic steps.
The target protein, a recombinant 26-kDa zinc metalloenzyme (see the “Properties” box), was expressed in E. coli at high yield (∼4 g/L) as a soluble, intracellular protein. Protein quantity and purity were determined using sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Cells were harvested by centrifugation, then resuspended and homogenized by three passes in a high-pressure homogenizer at 4 °C. The extract was clarified with polyethyleneimine (PEI) and centrifuged again.
Initial Capture Strategies
Considering the very high isoelectric point predicted from the target protein’s amino acid sequence (pI ∼10.5), our obvious choice for the capture step was cation-exchange chromatography. However, the protein was not soluble in the crude extract at the low ionic strength needed for IEX binding. Although the presence of 150-mM NaCl in the solution prevented precipitation of the target protein, capture by ion-exchange chromatography at this ionic strength was poor (∼50%) at pH 5–7 on several cation exchangers. We then attempted to recapture the target protein from the flow-through fractions. Although yields were considered acceptable for preliminary studies, for large-scale manufacture we considered this recapture step to be undesirable.
SOME BRANDS OF MIXED-MODE SORBENTS FOR BIOPROCESSING
Capto Adhere, Capto MMC (GE Healthcare)
Hydroxyapatite (e.g., CHT ceramic from Bio-Rad Laboratories, Fast Flow crystalline from Calbiochem, and HA-Ultrogel crystalline from Pall Life Sciences)
MEP HyperCel, HEA HyperCel, and PPA HyperCel (Pall Life Sciences)
PolyABX and PolyCSX (JT Baker)
PROPERTIES OF TARGET PROTEIN
The target protein is a soluble intracellular zinc metalloenzyme with no cysteines. Its molecular weight is 26 kDa, and its isoelectric point (pI) is 10.5. The protein loses activity at >∼pH 7.2.
We next evaluated capture from crude feedstock using immobilized metal-affinity chromatography (IMAC). IMAC sorbents charged with zinc, nickel, copper, and cobalt were evaluated, with the highest level of capture observed used a copper-charged IMAC column. However, enzymatic activity of the eluted target protein was significantly reduced by this procedure. Furthermore, we found the concentrated solutions to be blue, which suggests incorporation of copper into the protein, possibly through exchange of its zinc ion. Therefore, IMAC was ruled out.
We also evaluated hydrophobic-interaction chromatography (HIC) for the capture step. Screening studies using partially purified protein were performed using an HIC test kit from GE Healthcare, with the best capture obtained on a Phenyl Sepharose Hi Sub sorbent at 0.5-M (NH4)2SO4, pH 6.9. However, we observed that the target protein was not tightly bound because it slowly migrated down the column and began eluting during the loading process. In consequence, we increased the concentration of (NH4)2SO4 to 0.8 M. In trials with partially purified protein, this concentration led to tight binding to the Phenyl Sepharose, but addition of 0.8-M (NH4)2SO4 to the clarified crude feedstock led to precipitation. The precipitate contained a significant proportion of target protein, which represented unacceptable product loss.
What Worked — and Why
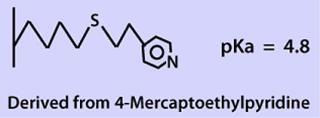
Studies using conventional HIC sorbents suggested that a more hydrophobic sorbent might be effective. Our search led us to evaluate the MEP HyperCel mixed-mode sorbent from Pall Life Sciences (www.pall.com). Its ligand includes a thioether-linked heterocyclic ring, 4-mercaptoethylpyridine (MEP, Figure 1). According to the manufacturer’s product literature, the ligand density specification is 70–125 µM/mL of sorbent, a significantly higher degree of substitution than for most HIC sorbents and substantially higher than even Phenyl Sepharose Hi Sub (40 µM/mL sorbent).
Figure 1: ()
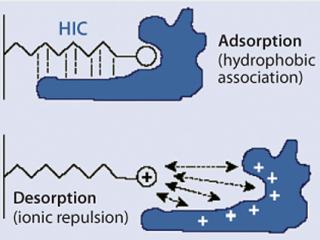
Figure 2 illustrates the mechanism of protein binding and elution on MEP HyperCel sorbent. Under near-neutral conditions, the pyridine ring is uncharged and hence hydrophobic. As the pH of the mobile phase is reduced and approaches the ligand’s pKa (4.8), the net positive charge increases on the ligand, as does the net positive charge on the bound protein. Ultimately, desorption is prompted by electrostatic charge repulsion, so high recovery of bound protein is achieved despite the high density of hydrophobic ligand on the sorbent. Based on hydrophobic-interaction binding and the pH-induced transition of the ligand from an uncharged to a charged form, this form of mixed-mode chromatography is referred to as hydrophobic charge-induction chromatography or HCIC (1).
Figure 2: ()
Although the target protein in our study is a nonantibody, it is noteworthy that immunoglobulins bind to the MEP HyperCel sorbent through a combination of hydrophobic and affinity interaction. Specifically, antibodies have been shown to have an affinity for ligands bearing nitrogen heterocycles and thioether structures (2). Accordingly, this mixed-mode sorbent has been used as a substitute for protein A in antibody capture (3, 4). It has also been used effectively as a polishing step in Protein A–based schemes (5). Because of the combined hydrophobic and affinity interaction, antibodies typically bind to the sorbent without addition of binding-promoting salts (6, 7).
By contrast, nonantibody proteins typically bind solely through hydrophobic interaction, promoted by the addition of a lyotropic salt such as (NH4)2SO4 or in some cases NaCl. However, because of the high ligand density of this sorbent, protein binding is typically accomplished at a lower salt concentration than what is required with conventional HIC sorbents. Indeed, binding of our target protein on MEP HyperCel media required addition of 0.5-M (NH4)2SO4, which was less than the 0.8 M (NH4)2SO4 concentration required for tight binding on Phenyl Sepharose Hi Sub media. Moreover, binding was maintained even when, following loading, the column was washed with (NH4)2SO4-free equilibration buffer. This is in marked contrast to typical behavior using conventional hydrophobic-interaction sorbents. Indeed, as described more fully below, a preelution wash with an (NH4)2SO4-free buffer at incrementally reduced pH values prompted desorption of a mixture of impurity components but little or no desorption of the target protein (Figure 5 and Figure 6).
Before proceeding to our findings, consider two related mixed-mode sorbents in relation to the one we chose (see the “Some Brands” box): HEA HyperCel and PPA HyperCel media. These two operate by mixed-mode hydrophobic-interaction chromatography rather than by HCIC. The distinction is a matter of chromatographic mechanism. For HEA and PPA HyperCel brands, the ligand does not typically undergo transition from an uncharged to a charged form during binding and elution.
The PPA ligand includes a phenylpropyl amine structure, whereas the HEA ligand includes a hexyl amine structure. The pKa is ∼8 for both. Thus, under the near-neutral to mildly alkaline conditions typically used during binding (e.g., pH 6.5–8.5), these ligands carry a significant net positive charge. For both sorbents, elution is prompted through electrostatic charge repulsion by reducing the pH into the range of 3–5 (8), which confers a net positive charge on the protein and causes desorption.
If suppression or prevention of ion-exchange binding contributions is desired during loading, 150-mM NaCl can be included in the buffer. To accomplish binding of very basic proteins, charge-repulsion effects generally can be overcome by the addition of a lyotropic salt and/or operation at increased pH (e.g., pH = 9). Except in such cases, binding is typically accomplished without need for addition of lyotropic salt. As with MEP HyperCel media, binding in the absence of added salt is accomplished based on the high ligand density of the sorbents.
Taken together, these three mixed-mode sorbents provide a useful range of retentivity and selectivity characteristics. As a practical matter, PPA HyperCel sorbent is the most retentive of the three and MEP HyperCel sorbent is typically the least retentive.
Determination of Binding Capacity
To determine the binding capacity of MEP HyperCel sorbent for our target protein, we performed a loading study using the E. coli feedstock. The column was equilibrated with 25-mM sodium phosphate buffer and 0.5-M (NH4)2SO4 at pH 7.2. The PEI-treated, clarified extract containing 2.9 g/L of target protein was made 0.5 M in (NH4)2SO4. After adjustment to pH 7.2, the feedstock was filtered to remove trace particulate matter and then loaded onto the column at a flow rate that provided a five-minute residence time. Breakthrough occurred after ∼98 g/L had bound (Figure 3).
Figure 3: ()
Optimization of Washing and Elution Steps
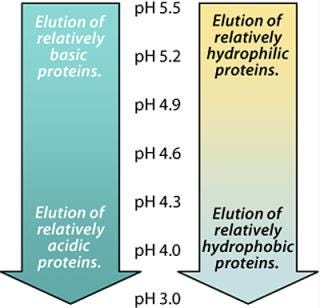
During our initial studies, the target protein was eluted from MEP HyperCel sorbent at pH 4.0. The eluent was cloudy and <50% pure. We achieved more selective desorption of the target protein and recovery of higher-purity product by optimizing the elution pH, as described by Schwartz, et al. (9). We also paid careful attention to the ionic strength of washing and elution buffers, as recommended by Weatherly et al. (10). Figure 4 illustrates the principles underlying our optimization based on elution pH.
Figure 4: ()
As noted above, the pyridine ring will take on a net positive charge as pH is reduced and approaches the pKa value of the MEP ligand. For example, at pH 5.8, the ligand will carry a 10% net positive charge. As illustrated in the Figure 4, basic proteins will tend to elute at higher pH values than will acidic proteins. Similarly, hydrophilic proteins will tend to elute at higher pH values than will hydrophobic proteins. Because our target protein was both basic and relatively hydrophilic, we expected it to elute at a higher pH than many of the host cell proteins, which tend to be acidic or hydrophobic.
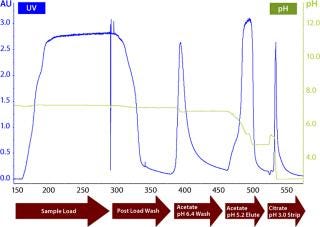
By sequentially washing the MEP HyperCel column with buffers of progressively lower pH, we determined the lowest value at which our target protein remained bound and the highest pH at which it eluted. Washing at the lowest feasible pH removed weakly binding impurities (Figures 5 and 6), with a preelution wash at pH 6.4. Eluting the target protein at the highest feasible pH facilitated retention of more strongly binding impurities (Figures 5 and 6), with elution of the target fraction at pH 5.2. A mixture of strongly binding impurities is desorbed at pH 3.0.
Figure 5: ()
Mixed-mode chromatography on MEP HyperCel media thus achieved both capture and a significant degree of purification in one step. Moreover, binding was achieved at a salt concentration lower than that required for conventional HIC and within a concentration range that prevented protein precipitation. Equally important, the target fraction was recovered in dilute buffer at a pH value optimized for selective desorption of the target protein. Using conventional HIC, the target fraction is frequently collected in a mobile phase that contains a significant concentration of lyotropic salt. In such cases, extensive diafiltration may be required in advance of the next unit operation.
Scale-Up: We initially developed our process using a 1-mL column. When scaled to 15 L, the only adjustment needed to widen the wash/elution window (increasing the wash pH and decreasing the elution pH by 0.2 pH units) to make process specifications less stringent.
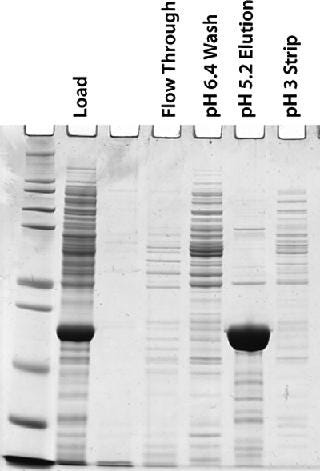
Five 100-L fermentations produced about 4 g/L of our target protein for purification. The PEI-clarified extract was made 0.5 M in (NH4)2SO4 and adjusted to pH 7.2. It was then loaded onto a 15-L MEP HyperCel column equilibrated with 25-mM sodium phosphate and 0.5 M (NH4)2SO4 at pH 7.2. Figure 5 shows a representative chromatogram, and Figure 6 shows a representative SDS-PAGE analysis of the feedstock and chromatographic fractions. We saw little or no target protein in the flow-through or wash fractions. Virtually all our target protein eluted at pH 5.2. Step yields of 75–95% were obtained in five separate runs, with increasing yields obtained as we gained experience during successive runs. In all cases, purity was >95%.
Figure 6: ()
Finally, use of a preelution wash step at pH 6.4 is noteworthy because the MEP ligand would be expected to carry little net positive charge at this pH value, given that the pKa of the ligand is 4.8. Desorption of impurities thus would not be prompted by charge repulsion. We speculate that the impurity components desorbed at pH 6.4 may be rich in histidine. The histidine imidazole group has a pKa of ∼6. At pH 6.4, histidine imidazole groups would be protonated to a significant extent. In protonated form, they would display reduced hydrophobic characteristics and might be expected to facilitate desorption of histidine-rich impurities. So our findings suggest that a preelution wash step in this pH range may be generally useful.
ADVANTAGES OF MIXED-MODE CHROMATOGRAPHY
Broadly, by combining multiple modes of interaction in a single sorbent, mixed-mode chromatography offers unique selectivities and the potential for reducing the number of chromatographic steps in scheme. A sorbent ligand may combine, for example, hydrophobic, ion-exchange, and pi-pi interactions. Specifically, in the case study presented here, binding was achieved at reduced salt concentrations compared with conventional HIC and allowed for product recovery in dilute buffer, reducing the need for intermediate diafiltration.
MEP HyperCel and Endotoxin
The capture chromatography step with MEP HyperCel reduced endotoxin levels by more than 1,000-fold down to ∼0.3 endotoxin units/mg protein, which was within the tolerance set for the final product. Endotoxins are both hydrophobic and acidic, so they would be expected to remain bound to the sorbent, which is positively charged and hydrophobic at the elution pH. Indeed, buffer conditions that promote desorption of the recombinant protein through charge repulsion will cause LPS to bind more tightly to the positively charged ligand. This suggests that MEP HyperCel sorbent generally should be effective for endotoxin clearance.
Nucleic acids should also be significantly reduced by mixed-mode sorbents containing a positive charge. Any DNA that does not flow through would be retained on the sorbent under conditions used to prompt elution of the target protein. In the case of MEP media, lowering the pH to promote elution establishes a net positive charge on the ligand, increasing binding of the nucleic acid. Very large reductions in nucleic acid levels have been reported by Ferriera et al. using this sorbent (4).
A Successful Case
MEP HyperCel mixed-mode sorbent provided effective purification of our recombinant therapeutic protein, for which other sorbents were unsuccessful. We achieved >95% purity, with 75–95% recovery during the capture step alone. Furthermore, this capture step reduced endotoxin to clinically acceptable levels, even though the feedstock was challenging (a clarified E. coli extract). Significantly, binding was achieved with lower (NH4)2SO4 concentration than what was required using conventional HIC. At this lower salt concentration, product precipitation and/or loss did not occur, rendering mixed-mode chromatography practical where conventional HIC was not. Moreover, the target fraction was recovered in dilute buffer, reducing or eliminating the need for diafiltration following this chromatographic step. Given their advantages (as described in the “Advantages” box), we predict that mixed-mode sorbents such as this will find broad application in the quest to rapidly develop cost-effective purification schemes for therapeutic proteins.
REFERENCES
1.) Burton, S, and D. Harding. 1995. Hydrophobic Charge Induction Chromatography: Salt Independent Protein Adsorption and Facile Elution with Aqueous Buffers. J. Chromatogr. A 814:71-81.
2.) Scholz, GH. 1998. Salt-Independent Binding of Antibodies from Human Serum to Thiophilic Heterocyclic Ligands. J. Chromatogr. B Biomed. Sci. Appl. 709:189-196.
3.) Ghose, S. 2006. Evaluation and Comparison of Alternatives to Protein A Chromatography Mimetic and Hydrophobic Charge Induction Chromatographic Stationary Phases. J Chromatogr. A1 122:144-152.
4.) Ferreira, G. 2007. A Two-Column Process to Purify Antibodies Without Protein-A. BioPharm Int..
5.) Chen, J, J Tetrault, and A. Ley. 2008. Comparison of Standard and New Generation Hydrophobic Interaction Chromatography Resins in the Monoclonal Antibody Purification Process. J. Chromatogr. A 1177:272-281.
6.) Schwartz, W. 2001. Comparison of Hydrophobic Charge Induction Chromatography with Affinity Chromatography on Protein A for Harvest and Purification of Antibodies. J. Chromatogr. A 908:251-263.
7.) Guerrier, L. 2000. New Method for Selective Capture of Antibodies Under Physiological Conditions. Bioseparation 9:212-221.
8.) Brochier, VB. 2008. Fast Purification Process Optimization Using Mixed-Mode Chromatography Sorbents in Prepacked Mini-Columns. J. Chromatogr. A 1177:226-233.
9.) Schwartz, W. 2004. Application of Chemically Stable Immunoglobulin-Selective Sorbents: Capture and Purification of Antibodies with Resolution of Aggregate. Bioprocessing J.:53-62.
10.) Weatherly, G. 2002. Initial Purification of Recombinant Botulinium Neurotoxin Fragments for Pharmaceutical Production Using Hydrophobic Charge Induction Chromatography. J. Chromatogr. A 952:99-110.
11.) Arakawa, T. 2009. MEP Chromatography of Antibody and Fc-Fusion Protein Using Aqueous Arginine Solution. Prot. Express. Purif. 63:158-163.
12.) Zhao, G, and Y. Sun. 2007. Displacement Chromatography of Proteins on Hydrophobic Charge Induction Adsorbent Column. J. Chromatogr. A 1165:109-115.
13.) Bak, H, and ORT. Thomas. 2007. Evaluation of Commercial Chromatographic Adsorbents for the Direct Capture of Polyclonal Rabbit Antibodies from Clarified Antiserum. J. Chromatogr. B 848:116-130.
14.) Dux, MP. 2006. Purification and Scale-Up of a Recombinant Heavy Chain Fragment C of Botulinum Neurotoxin Serotype E in Pichia pastoris GS115. Prot. Express. Purif. 45:359-367.
15.) Mowry, MC. 2004. Production and Purification of a Chimeric Monoclonal Antibody Against Botulinum Neurotoxin Serotype A. Prot. Express. Purif. 37:399-408.
16.) Coulon, D. 2004. Penicillin Acylase Purification with the Aid of Hydrophobic Charge Induction Chromatography. J. Chromatogr. B 808:111-115.
17.) Boschetti, E. 2001. The Use of Thiophilic Chromatography for Antibody Purification: A Review. J. Biochem. Biophys.Meth. 49:361-389.