Biopharmaceutical manufacturing is divided into two areas: upstream fermentation or cell culture and downstream purification processes. Each area contains multiple unit operations. A unit operation is defined as a step in processing using a particular type of equipment. Here, we focus on downstream process development, which must reliably produce a highly purified drug substance (often >99%). Downstream processing includes recovery, capturing, and polishing steps.
The primary downstream unit operation is chromatography because of its simplicity and high resolving power (1). Most process trains involve at least two distinct, orthogonal chromatography steps chosen from the number of chromatography supports available with various ligands attached to solid matrix. Affinity, cation-exchange (CEX), anion-exchange (AEX), ceramic hydroxyapatite (CHT), and hydrophobic-interaction chromatography (HIC) are the main types of chromatography modes used in large-scale bioseparations. The mode used for polishing depends mainly on the nature of the impurities (2). Chromatography can be operated in bind-and-elute (e.g., during the initial capture on protein A resin in antibody manufacturing) or flow-through mode (e.g., during the contaminant removal with AEX membrane adsorbers or resins) (3, 4). For monoclonal antibody purification, AEX and HIC are often operated using flow-through mode, in which the product does not bind to the chromatography support but specific contaminants are retained (2).

Figure 1:
SARTORIUS-STEDIM (WWWSARTORIUS-STEDIM.COM)
In current biomanufacturing processes, which emphasize speed-to-market for business success, a standardized platform approach to downstream process development is applied to reduce time to clinic and improve process economics (2, 5, 6). Downstream costs account for nearly 50% of total manufacturing costs (6). Therefore, during process development, techniques are evaluated that reduce process complexity, buffer consumption, labor, and time — while still producing the required high-quality product. Furthermore, higher upstream productivity has prompted discussion on increasing throughput in downstream unit operations (7, 8).
Gottschalk, Thoemmes, and Etzel evaluated alternative formats for recovery and purification unit operations with nonchromatographic formats and membrane chromatography (7, 8). Given these requirements, a novel membrane adsorber was developed for large-scale purification of biomolecules based on HIC principles. A macroporous membrane structure was designed for high flow rates and binding capacities. Using the advantages of membrane chromatography technology, the novel Sartobind phenyl membrane can be considered as an alternative to resins for applications in which throughput and processing time need improvement.
Advantages of Membrane Chromatography Technology
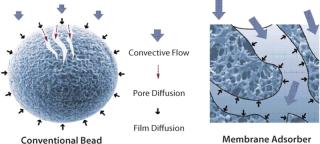
Membrane chromatography is amenable to large-scale purification of biotherapeutic products and has been part of a commercial, FDA-approved process for DNA and virus clearance since 2001 (9). The performance of disposable membrane chromatography makes it suitable as a platform technology in large-scale biomanufacturing. A significant functional advantage of membranes over resins is that the transport of molecules to their binding sites takes place mainly by convection with minimal pore diffusion (Figure 1) (10), which results in a binding capacity more or less independent of the flow rate (11). The flow-rate limitation associated with HIC column chromatography can increase the risk of protein degradation because of long contact time on the hydrophobic surface. Moreover, because of their hydrodynamic benefits, flow-through processes based on membrane chromatography involve much smaller devices than columns with a similar throughput. This consequently reduces buffer consumption, processing time, and space requirements. Flow-through membrane chromatography can reduce ≤95% of buffer consumption and 66% of processing time (4). The advantages and performance of membrane chromatography have been well described (12,13,14,15).
Figure 1: ()
Membrane adsorbers come in reusable form as well as a fully disposable technology that eliminates the need for reuse validation at large scales (10, 16). Our company has developed a Sartobind HIC membrane adsorber as detailed below.
Hydrophobic-Interaction Membrane Chromatography
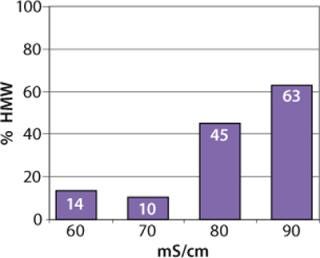
HIC is used to purify molecules based on differences in their hydrophobicity. It is an efficient mode to remove dimers and high—molecular-weight aggregates in a monoclonal antibody purification process as a polishing step (17). HIC has evolved into one of the most powerful methods in preparative biochemistry (18,19,20,21). It has also been described as a method of clearing multiple product-related impurities in Fc fusion protein purification (22).
HIC separation conditions are opposite to those applied in ion-exchange chromatography. A sample is loaded at high salt concentration, which promotes the interaction between its hydrophobic surface and the hydrophobic surface of an adsorbent (23). Most proteins adsorbed are effectively eluted by washing the adsorbent with pure water or low–ionic-strength buffer solution (24, 25). The main parameters to consider when selecting an HIC adsorbent and optimizing separation processes on such media include ligand type and the degree of substitution, the type and concentration of salt, pH, temperature, additives, and the type of base matrix. It is important not to overlook the contribution of the base matrix.
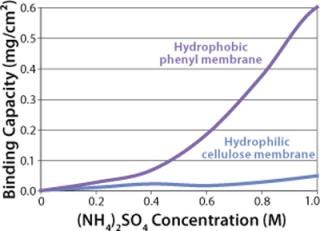
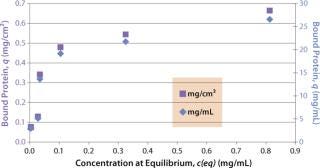
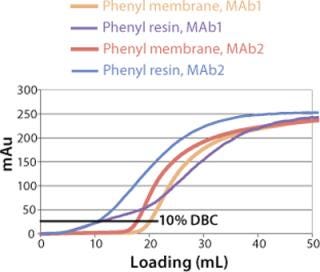
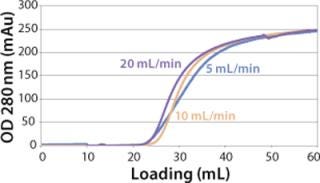
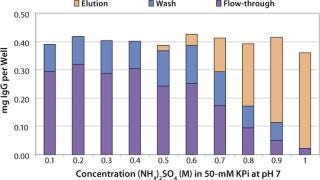
Sartobind HIC membrane adsorbers are based on hydrophilic, regenerated, stabilized cellulose with hydrophobic phenyl groups covalently attached to the base matrix. We compared the effect of concentration of ammonium sulfate in 50 mM potassium phosphate buffer at pH 7 on the static binding capacity for a human polyclonal antibody on the phenyl membrane with that of the base cellulose membrane without phenyl functional groups (Figure 2). Our results demonstrate that the nonspecific interaction from the cellulose base matrix is negligible. Properly combining the new membrane structure with hydrophobic phenyl functional groups resulted in a novel hydrophobic convective permeable matrix. A high binding capacity in the range of chromatography resins is observed even at significantly higher flow rates (Figure 3A and B). And the examples presented in Figures (4567) illustrate similar hydrophobic-interaction mechanisms of protein binding as observed from the conventional resin materials. Figure 4 shows a load of two monoclonal antibodies on a membrane device and on a resin column at specified flow rates.
Figure 2: ()
Figure 3a: ()
Figure 3b: ()
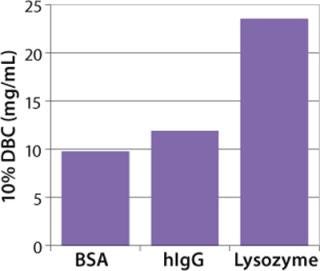
Figure 4: ()
Figure 5: ()
Figure 6: ()
Figure 7: ()
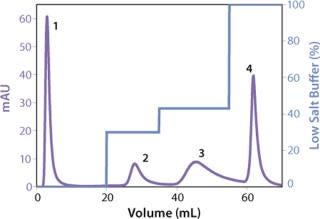
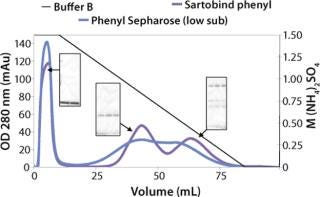
We observed no significant changes in dynamic binding capacities at various flow rates (Figure 5). This reflects the negligible diffusion limitation of mass transfer within the membrane macropores. High binding capacities and flow rates allow the use of the novel phenyl membrane adsorber for bind-and-elute and flow-through operations. Figure 6 shows a separation of a model protein mixture at flow rate of 10 mL/min.. After loading at 1.5 M ammonium sulfate concentration on a Sartobind phenyl MA 75 (2.1 mL membrane volume, 15 layers), the proteins (cytochrome c, myoglobin, lysozyme and α-chymotrypsinogen) were eluted when we applied a step gradient to low ionic strength in order of their increasing hydrophobicity. Protein recovery of >90% was obtained (data not shown). Figure 7 presents the separation profiles of cytochrome c, trypsinogen, and polyclonal antibody on phenyl membrane and low-sub phenyl Sepharose from GE Healthcare (www.gehealthcare.com) while applying a linear gradient. The inserts show SDS-PAGE analytics of the elution fractions to verify the nature and the purity of the protein. And Figure 8 illustrates an application of the phenyl membrane in flow-through mode for aggregate removal in a purification process for a recombinant protein. We chose the loading conditions to selectively retain the high–molecular-weight aggregates, whereas the target protein did not bind to phenyl membrane. The amount of aggregates bound on the membrane increased with higher ammonium sulfate concentrations in the loading buffer (binding conditions were not optimized).
Figure 8: ()
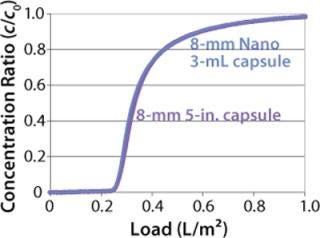
We also incorporated the membrane into 96-well plates and spin columns to investigate binding conditions and yield with a model antibody. The well-plate format allowed for high-throughput screening of process conditions (Figure 9). For large-scale process applications, the hydrophobic membrane is assembled into 30-layer radial flow process capsules with an 8-mm bed height. The capsules have the same design and housing materials as Sartobind IEX capsules used in various downstream processes for removal of contaminants (4)(26,27,28) or for purification of large biomolecules and viruses (29). Scale-up consideration of Sartobind nano and 5-in. capsules showed excellent conformity to a 42-fold scale up, as demonstrated by normalized breakthrough curves in Figure 10.
Figure 9: ()
Figure 10: ()
Principal HIC use on membranes is comparable to that on beads. In all applications, it is advisable to optimize by moderating temperature, pH, salt concentration, and type of salt. The newly designed membrane structure in combination with appropriate hydrophobic surface functionality provides a scalable and disposable tool for purification and separation of biomolecules, as well as for polishing applications in the biopharmaceutical industry. One preferred application of membranes is in flow-through steps. The ready-to-use capsule format requires no packing or testing and allows for a “plug-and-play” approach in downstream processing.
The Sartobind phenyl membrane can be considered for capturing large molecules and unstable molecules that need to be rapidly processed. Whereas flow-through columns are designed based on flow rate rather than binding capacity, Sartobind phenyl membrane provides a robust alternative to flow-through columns for the removal of hydrophobic contaminants. Misfolded proteins, host-cell proteins, aggregates, dimers, trimers, tetramers of biomolecules, and leached chromatographic ligands can display higher hydrophobicity than the protein of interest. Such impurities are specifically retained while the target protein can flow through.
Sartobind Q membrane chromatography has already proven to be a robust alternative to polishing Q column in flow-through mode for contaminant removal. In purification processes of monoclonal antibodies, protein A chromatography can yield >98% product purity in one step. Directly after a protein A column, a flow-through HIC membrane can be implemented as a polishing step to remove hydrophobic contaminants. This step can reduce the amount of leached protein A, and misfolded proteins and will supply additional safety to the product through orthogonal strategies such as HIC potency for viral clearance and the removal of hydrophobic process impurities (e.g., detergents and additives) (30, 31).
The evolution of downstream processes should focus on polishing with disposable technologies, which can reduce a downstream process to one column step purification (32). Combining a Sartobind Q membrane with hydrophobic-interaction membrane chromatography, as orthogonal chromatography steps for polishing, could reduce contaminants to acceptably low levels and ensure product safety. This membrane-based technology fulfills the requirements for high-throughput production while reducing process time and complexity (no packing and packing testing) and adding flexibility to downstream processes.
REFERENCES
1.) Curling, J, and U Gottschalk. 2007. Process Chromatography: Five Decades of Innovation. BioPharm Int. 20:10-19.
2.) Schukla, A. 2007. Downstream Processing of Monoclonal Antibodies: Application of Platform Approaches. J. Chromatogr B. Analyt. Technol. Biomed. Life Sci 848:28-39.
3.) Desai, MA Desai, AM. 2000.Application of Chromatography in the Downstream Processing of Biomolecules Downstream Processing of Proteins: Methods and Protocols:73-94.
4.) Zhou, JX, and T Tressel Basic Concepts in Q Membrane Chromatography for Large-Scale Antibody Production. Biotechnol. Prog. 22:341-349.
5.) Low, D, R O’Leary, and NS Pujar. 2007. Future of Antibody Purification. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 848:48-63.
6.) Sommerfeld, S, and J Strube. 2005. Challenges in Biotechnology Production: Generic Processes and Process Optimization for Monoclonal Antibodies. Chem. Eng. Process. 44:1123-1127.
7.) Gottschalk, U 2008. Bioseparation in Antibody Manufacturing: The Good, The Bad, and The Ugly. Biotechnol. Prog..
8.) Thoemmes, J, and M Etzel. 2007. Alternatives to Chromatographic Separations. Biotechnol. Prog. 23:42-45.
9.) Galliher, P, and E Fowler. 2001.Validation of Impurity Removal By the CAMPATH-1HIBC’s Biopharmaceutical Production Week, San Diego, CA. IBC Life Sciences, Westborough.
10.) Gottschalk, U, S Fischer-Fruehholz, and O Reif. 2004. Membrane Adsorbers: A Cutting-Edge Process Technology at the Threshold. BioProcess Int. 2:56-65.
11.) Knudsen, HL. 2001. Membrane Ion-Exchange Chromatography for Process-Scale Antibody Purification. J. Chromatogr. A 907:145-154.
12.) Lim, JAC. 2007. Economic Benefits of Single-Use Membrane Chromatography in Polishing. BioProcess Int. 5:60-64.
13.) Mora, J. 2006. Disposable Membrane Chromatography: Performance Analysis and Economic Cost Model. BioProcess Int. 4:S38-S43.
14.) Gosh, R 2002. Protein Separation Using Membrane Chromatography: Opportunities and Challenges. J. Chromatogr. A 952:13-27.
15.) Fraud, N 2008. Membrane Chromatography: An Alternative to Polishing Column Chromatography. Bioprocessing J. 7:34-37.
16.) Zhou, JX, T Tressel, and S Guhan. 2007. Disposable Chromatography: Single-Use Membrane Chromatography As a Polishing Option During Antibody Production Is Gaining Momentum. Biopharm Int.:25-33.
17.) Li, F. September–October 2005. Current Therapeutic Antibody Production and Process Optimization. Bioprocessing J.:1-8.
18.) Gagnon, P, E Grund, and T Lindback. 1995. Large-Scale Process Development for Hydrophobic-Interaction Chromatography, Part 1: Gel Selection and Development of Binding Conditions. LC-GC 13:318-328.
19.) Gagnon, P, E Grund, and T Lindback. 1995. Large-Scale Process Development for Hydrophobic-Interaction Chromatography, Part 2: Controlling Process Variation. LC-GC 13:400-410.
20.) Gagnon, P, and E Grund. 1996. Large-Scale Process Development for Hydrophobic-Interaction Chromatography, Part 3: Factors Affecting Capacity Determination. BioPharm 9:34-39.
21.) Gagnon, P, and E Grund. 1996. Large-Scale Process Development for Hydrophobic-Interaction Chromatography, Part 4: Controlling Selectivity. BioPharm 9:54-64.
22.) Evans, DRH. 2008. Purification of an Fc-Fusion Biologic: Clearance of Multiple Product-Related Impurities By Hydrophobic-Interaction Chromatography. J. Chromatogr. A 1177:265-271.
23.) Tiselius, A 1948. Adsorption Separation By Salting Out. Arkiv. Kemi. Mineralogi. Geologi. 26B:1-5.
24.) Porath, J. 1973. Salting Out in Amphiphilic Gels As a New Approach to Hydrophobic Adsorption. Nature 245:465-466.
25.) Hjerten, S, J Rosengren, and S Pahlman. 1974. Hydrophobic-Interaction Chromatography. J. Chromatogr. A 101:281-288.
26.) Zhou, JX. 2006. New Q Membrane Scale-Down Model for Process-Scale Antibody Purification. J. Chromatogr. A 1134:66-73.
27.) Clutterbuck, A, J Kenworthy, and J Liddell. 2007. Endotoxin Reduction Using Disposable Membrane Adsorption Technology in CGMP Manufacturing. BioPharm Int.:11-14.
28.) Arunakumari, A, J Wang, and G Ferreira. 2007. Improved Downstream Process Design for Human Monoclonal Antibody Production. BioPharm Int.:6-10.
29.) Specht, R. 2004. Densonucleosis Virus Purification By Ion-Exchange Membranes. Biotechnol Bioeng. 88:465-473.
30.) Omar, A, and H Koblet. 1988. Semliki Forest Virus Particles Containing Only the E1 Envelope Glycoprotein Are Infectious and Can Induce Cell–Cell Fusion. Virology 166:17-23.
31.) Huyghe, BG. 1995. Purification of a Type 5 Recombinant Adenovirus Encoding Human p53 By Column Chromatography. Hum Gene Ther. 6:1403-1416.
32.) Zhou, JEffective Incorporation of Disposables into Mab DSP BioProcess International European Conference and Exhibition, IBC Life Sciences, London.