According to an FDA guidance document, process analytical technology (PAT) tools “are intended to support innovation and efficiency in pharmaceutical development” (1). The agency encourages manufacturers to use a PAT framework for developing and implementing effective innovative approaches in development, manufacturing, and quality assurance. The sensors described here are one possible response to the requirement of systems by analyzing and controlling critical cultivation parameters with real-time process measurements.
Working Principle
Glucose was measured using a #CITSens Bio sensor (C-#CIT) (2, 3). It uses an enzymatic oxidation process and direct electron transfer from glucose to the electrode (anode) by a chemical wiring process. By contrast with other enzymatic glucose sensors, it works without cross-sensitivity to oxygen. To separate the sensor from the cultivation medium, a dialysis membrane is cast over the sensing head, which is delivered ready-to-use (gamma-irradiated and integrated into the ventilation cap of the shake flasks).
PRODUCT FOCUS: ALL BIOLOGICALS
PROCESS FOCUS: CELL CULTURE
WHO SHOULD READ: PROCESS DEVELOPMENT
KEYWORDS: PROCESS MONITORING, PROCESS CONTROL, CELL CULTURE MEDIA, CULTIVATION
LEVEL: INTERMEDIATE
The biosensor signal is read out by the connected water-tight beamer (Photo 1, blue) and wirelessly transmitted to a receiver (ZOMOFI, Albis Technologies). One ZOMOFI receiver can acquire the response of ≤1,000 sensors (cell culture vessels). Each sensor and Bio Beamer unit is identified by a specific tag to ensure correct data assignment. The receiver connects to a PC for data processing.
Photo 1:
Photo 1: l ()
We used an SFR shake flask reader (Photo 2, PreSens GmbH) in all experiments to monitor pH and dissolved oxygen concentration (4). The system applies chemical optical sensors attached to the bottom of the shake flasks. Those sensors change their fluorescence due to changes in pH and oxygen. The SFR tray mounts in the shaker and transmits data wirelessly to a PC or laptop computer.
Photo 2:
Photo 2: ()
Experiments, Biocompatibility, and Specifications
We mounted three gamma-irradiated glucose sensors from C-#CIT AG on three 125-mL shake flasks with integrated pO2 and pH sensor spots. We filled the flasks with cell culture media (ChoMaster HP-1, Cell Culture Technologies LLC)) and inoculated with CHO cells (5).
The starting viable cell density was 0.64 × 105 cells/mL at 97% viability Glucose concentration was 3.59 g/L as measured with an enzymatic off-line analyzer (BioProfile, Nova Biomedical Corp.), and 4.05 g/L measured with HPLC. For practical reasons, we calibrated the glucose sensors according to the enzymatic analyzer at 3.6 g/L. Calibration of the glucose sensors is a one-point calibration — made just after adding the cells by inserting the start concentration of glucose in the software interface of the #CITSens Bio sensor. Throughout, we analyzed the whole experiment’s samples off-line as well.
During the first four days, we incubated cells at 37 °C and produced a cell mass. On day 4, we exchanged cell culture medium to a production medium and reduced temperature to 31 °C to shift to protein production for the next six days.
Results and Discussion
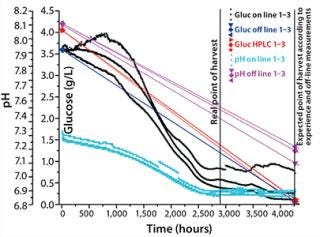
The starting concentration measured using high-performance liquid chromatography HPLC) was 4.05 g/L, whereas the starting concentration measured using an enzymatic analyzer was 3.59 g/L, a difference of 12.5% between the two reference methods (Figure 1). We calibrated the in-line glucose sensors according to the values of the enzymatic analyzer and therefore show 3.6 g/L at the beginning. After four days, one of the three glucose sensors showed slightly higher values. Nonetheless, all sensors detected the same glucose consumption. At the end of the run, one process sensor showed two high values (0.7 g/L instead of 0.3 g/L), but this is not really relevant because it clearly indicates the end of the process.
Figure 1: ()
The difference of ~0.5 g in initial values measured with the off-line methods — analyzer and HPLC — demonstrates one of the problems in glucose determination. Depending on the method used, sampling, and time differences until analysis, results can get falsified. It is questionable how suitable off-line methods are for process control, so online measurement might be preferable.
The same is evident in pH values. We meaured pH with an off-line method as well as with the pH sensor spots. The pH measured in off-line analysis was 8.1, and that measured with the chemical optical sensors was 7.2–7.3. The difference results not from an incorrect measuring method, but from different samples measured at different environmental conditions (incubator: 5% CO2 and 37 °C; laboratory environment: 0.04% CO2 and 21 °C).
We set the incubator atmosphere to a CO2 concentration of 5% v/v. CO2 needs to equilibrate in the medium, and the final pH is reached only after having the cell culture media in the incubator for a couple of minutes. Because the sample measured in off-line analysis was drawn from fresh medium, we did not adjust the pH to the incubator atmosphere. During cultivation, off-line pH values will therefore always be higher than the pH measured with in-line sensors. Even at the end of the four days, off-line pH was 7.2–7.3, whereas pH measured with in-line sensors was 6.7–6.8. However, the pH measured with in-line sensors always reflects the real situation, whereas o
ff-line methods do not allow measurement under real conditions.
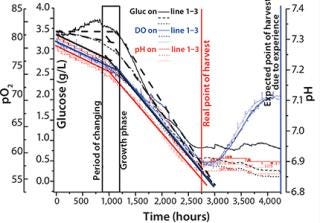
During the first four days, all three sensor types (pH, pO2, and glucose) showed the three typical growth phases of the cell cultures: lag, exponential, and stationary (Figure 2). Because of past experience and external sample analysis, we assumed that our cell culture needed at least 72 hours to reach the maximal cell density with a high level of living cells. That was not the case, as Figure 2 shows. The online sensors detected the optimal point of harvest after ~45 hours. Analysis showed that the used media changed slightly, leading to faster cell growth. That demonstrated the importance of online analysis: If a time-controlled process or manual sampling had been applied towards the end of the process, such changes might never have been discovered, and a great amount of production time would have been wasted.
Figure 2: ()
Considering the glucose consumption per hour, the different growth phases are clearly visible. In the first 10 hours, there was a clear lag phase in which the slope of all three sensors was different from the slope during the exponential growth phase that followed for the next 36–42 hours. In the following stationary phase, glucose and pH did not change, and O2 increased as a result of less O2 consumption at the same rotation speed.
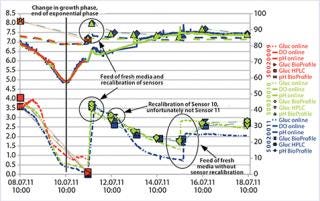
After the first four days, we exchanged the cell culture medium and used the cells to produce protein (Figure 3). The temperature in the incubator was decreased to 31 °C, and the glucose sensors were recalibrated. During the next six days, we drew daily samples for off-line pH analysis and took HPLC measurements.
Figure 3: ()
Off-line pH values had a slight offset compared with the online measured values using PreSens sensors. This offset decreased towards the end of cultivation time but nevertheless showed a difference of 0.3 pH units. Online oxygen measurement clearly displayed the increased oxygen uptake during the exponential growth phase. During the production phase, oxygen level in the culture media rose again, because oxygen transfer into the media exceeded oxygen consumption by the cells. The measured glucose values also indicate the different cultivation phases.
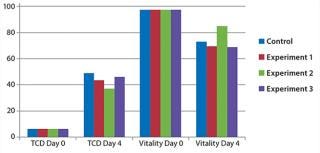
During the entire experiment, we determined the cell density and counted living cells. In that time, we observed no difference in cell density and amount of living cells compared with cell cultures without sensors (Figure 4).
Figure 4: ()
Advantages of Online Measurement
Examples described here demonstrate the effectiveness of continuous monitoring to control the quality of bioprocesses. In-line sensors offer several advantages such as elimination of sampling, reduced risk of contaminations, absence of reagents costs compared with batch analyzers, and immediate detection of contaminats or other process changes. Our results clearly indicate that online DO reading and glucose measurement precisely monitor metabolic changes in the culture. Both independently measured variables show the same point of harvest. Because of the intermittent nature of off-line measurements, these conventional methods can be as precise in terms of time-dependent analysis. We applied the presented monitoring tools to shake flasks. These tools can also be used for larger volumes such as single-use bioreactors.
About the Author
Author Details
Irina Bauer is a research associate and Iris Poggendorf is a lecturer at the Zurich University of Applied Sciences, School of Life Sciences and Facility Management, Institute of Biotechnology, Biochemical Engineering and Cell Cultivation Technique. Stefan Spichiger is general manager and Ursula E. Spichiger is president of the Board of Directors at C-CIT AG. Corresponding author Gernot Thomas John is director of marketing and Innovation at PreSens Precision Sensing GmbH, Josef-Engert-Str. 11, 93053 Regensburg, [email protected].
REFERENCES
1.) 2004. Guidance for Industry, Process Analytical Technology—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; Availability. Fed. Reg. www.gpo.gov/fdsys/pkg/FR-2004-10-04/pdf/04-22203.pdf 69:59257-59258.
2.) Spichiger, S, and UE. Spichiger-Keller. 2010.New Single-Use Sensors for Online Measurement of Glucose and Lactate: The Answer to the PAT InitiativeSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley and Sons, Inc, Hoboken.
3.) Spichiger, S, and UE. Spichiger-Keller. 2010. Process Monitoring with Disposable Chemical Sensors Fit in the Framework of Process Analysis Technology (PAT) for Innovative Pharmaceutical Development and Quality Assurance. Chimia 64:803-807.
4.) Schneider, K. 2009. Optical Device for Parallel Online Measurement of Dissolved Oxygen and pH in Shake Flask Cultures. Bioprocess Biosyst. Eng. 33:541-547.
5.) Mazur, X. 1999. Higher Productivity of Growth-Arrested CHO Cells Using a Novel Autoregulated Production Process, ETH-Zuerich.