Today’s renewed interest in perfusion culture is due to an increased awareness of its advantages, some general improvement in equipment reliability, and a broadening of operational skills in the biomanufacturing industry. Some misperceptions persist, however, according to a 2011 review by Eric Langer (1). Our view here of the history of perfusion and fed-batch processes includes some discussion of technological process improvements and challenges that the bioprocess industry faces.
A team of authors at Serono in Switzerland wrote in 2003:
The major advantage of the perfusion mode is high cell number and high productivity in a relatively small-size bioreactor as compared with batch/fed-batch. In order to sustain high cell number and productivity, there are needs to feed medium during the cell propagation phase and the production phase. In contrast to batch and fed-batch processes, where there is no metabolites removal, in continuous processes medium is perfused at dilution rates exceeding the cellular growth rate. For this, a good separation device is needed to retain cells in the bioreactor. (2)
Many cell retention devices perform well, to a greater or lesser degree, at small scale, including gravity-based cell settlers, spin filters, centrifuges, cross-flow filters, alternating tangential-flow filters, vortex-flow filters, acoustic settlers (sonoperfusion), and hydrocyclones. All are described well in the 2003 paper mentioned above. But only a few types are reliable at larger scales and scalable enough for bioindustrial use.
Perfusion performed at CMC Biologics ()
Here I compare the ATF System from Refine Technology with spin filters, cell settlers, and centrifuges. I am not including other technologies here because of scalability limitations and a lack of proven market acceptance.
Perfusion’s Early Potential
The advantages of using perfusion for enhancing production of cell-derived products were realized in the late 1980s and early 1990s. In those early days of the modern biotechnology industry, production cell lines were not fully developed, and their product expression was very small — from a few micrograms to a few hundred milligrams per liter in batch or fed-batch. Attainable cell concentrations were only a few million per milliliter.
Spin Filters: Perfusion offered a way to derive more product from such low producers. It was well known that perfusion could increase cell concentration by as much as an order of magnitude (3). The spin filter was the most common perfusion device used; it was the best cell-separating device available at the time, supported by reputable equipment manufacturers.
Spin filters remain in use at a few sites but have been largely phased out, largely because of their limited scale-up potential and unreliability: When a bioreactor’s volume scales up by the cube of its radius, the surface area of its spin filter screen scales by the square of its radius. An internal spin filter can take up a significant portion of production space within a vessel, and once its screen fouls, the run is terminated. An external production spin filter may solve this shortcoming, but it has drawbacks related to cost, maintenance and sterilization difficulties.
A more important factor behind the lackluster acceptance of perfusion in those early years was the rapid evolution of cell biology. New, more productive expression systems and improved media development permitted large increases in culture productivity; product concentrations were increasing from several hundred milligrams to about a gram per liter. Production needs could, therefore, be achieved with the well-understood fermentation technologies, batch and fed-batch. Scale up was accomplished simply by moving to bigger vessels.
The success of batch and, more important, fed-batch, not only inhibited the wider acceptance of spin filters, but also of other evolving cell-separation technologies. The difficulties associated with spin-filter operations and the undeveloped state of new perfusion technologies stigmatized the process. The dominance of fed-batch continued well into the next decade.
However, despite the dominance of fed-batch as an industry standard, perfusion continued to be championed. Perfusion offered an excellent solution for production with unstable proteins that could not remain in the toxic environment of an ever-deteriorating fed-batch culture. With perfusion, such products could be removed rapidly from a vessel and stored appropriately to preserve their stability. Many people chose perfusion to bypass constraints of space and cost factors. Furthermore, as culture productivity increased, and although it greatly benefited fed-batch processes, perfusion promised even greater output from a continuous culture.
So the use of perfusion never died; in fact, as the use of spin-filters declined, other cell separation devices slowly emerged. Those were based on filtration, gravity settling, and centrifugation. Continued development of numerous products that held out the promise of commercialization provided the driving force to experiment with new culture technologies. Occasionally a perfusion process, primarily one based on using spin-filters, cell settling, and centrifugation, was scaled to commercial production.
High-Concentrations Are a Game-Changer: From the early 2000s and particularly in the past few years another critical transition in biopharmaceutical manufacturing occurred. Further advancements in development of cell lines, expression systems, and media formulations resulted in an impressive ability to grow cells to very high concentrations and achieve product concentrations previously inconceivable. Using fed-batch as a reference, in the mid 1990s attainable cell concentrations were about 5 × 106 cells/mL, with record product concentrations of 1–2 g/L; today those are greater than 15 × 106 cells/mL, with product concentrations of up to 10 g/L. Although those concentrations are still not typical, they indicate where the field is heading. Those results are amplified by the use of perfusion, through which substantially higher cell concentrations and product output can be achieved (4,5).
Perfusion Returns to Manufacturing
A general lack of manufacturing capacity forecast at the beginning of this century was overcome through both biological innovation and engineering construction. Today’s overcapacity places most of the available space in the hands of relatively few companies. Even as some large biofacilities are mothballed, newer companies build modern facilities based on the latest technologies. Few organizations would now consider building a new, multiple–20,000-L bioreactor facility. Rising competition in the healthcare sector, whether through generics/biosimilars or multiple drugs with the same indication, requires the vast majority of biopharmaceutical products to be more easily produced in smaller and more flexible plants — even in multiple locations. New ultrahigh-density cell culture processes such as concentrated fed-batch and concentrated perfusion are well suited to this new manufacturing enviro
nment and facilitate a shift toward single-use technologies. That helps companies reduce both risk and capital investment, allowing them to delay making major facility decisions.
Microcarrier-based perfusion in a single-use ATMI bioreactor ()
So the face of biomanufacturing today is very different from that of just a decade ago. Nearly everyone uses perfusion in some way — from large biopharmaceutical companies such as Pfizer, Medarex, and Genentech (6,7,8) to small biotech and novel vaccine manufacturers such as CMC Biologics and Crucell (9,10). Outside the established biomanufacturing infrastructure, biosimilar and other relatively new biological manufacturers such as Biocon and A-Bio are also looking favorably on the perfusion model because of its associated cost efficiency. Perfusion is back.
Simplicity and reliability have long been key factors to consider in biologics production, especially where manufacturing involves high-value products in a large-batch environment. The industry is now being challenged as it moves forward to realize the much–touted “factory of the future,” which will incorporate several platform technologies. One such technology is certainly the adoption of disposables throughout production facilities.
Perfusion is a broad term, which many people may still view unfavorably. Although many, in fact, use perfusion at some level, not everyone admits to it — nor to how they do it, nor how often. Companies are experimenting with perfusion to solve challenges or implement novel solutions at many process stages: high density, large-volume cell banking (11); seed expansion (8); n–1 perfusion (12); and even final production reactors (13). Perfusion has evolved too: It is no longer solely a two- or three-month process, but can be as short as a three-day boost to a standard fed-batch process. Perfusion has become a specialist operation. Implementation depends on the nature of different facilities, cell lines, processes, and products — as well as each company’s own operating philosophy. Success depends on many factors, not least of which is a company’s choice of perfusion system. But one challenge — that of producing a reliable cell-retention device — may have been solved to a great degree by a relatively new hollow-fiber perfusion device
Case Study
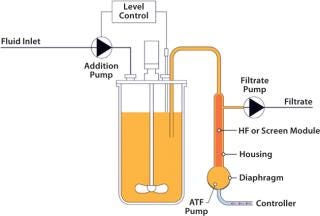
The ATF System (Figure 1) offers nearly linear scale-up for simplicity of operation and validation. Generally, conventional filtration systems will fail rapidly when used to separate media from a complex suspension of a cell culture with a high bioburden. By contrast, this particular system, due to its flow dynamics, has an inherent self-cleaning ability to allow its range of filter materials and pore sizes to perform significantly longer than might otherwise be expected.
Figure 1: ()
A standard hollow-fiber module is used to separate cells and product. However, unlike systems that recirculate a culture through a filter in one direction, the alternating tangential-flow action constantly cleans the fibers every five to 10 seconds with a backflush action. With only a single connection to the bioreactor, cells and media enter and leave the ATF system, flowing reversibly through the hollow fibers. Flow is controlled by the diaphragm moving up and down in the ATF system’s pump. This generates a rapid low-shear flow between vessel and pump, ensuring rapid exchange and prompt return of cells to the reactor and minimizing their residence outside the bioreactor. The choice of pore size for the hollow fiber determines what elements are retained and which ones pass through to the permeate (perfusion or concentrated fed-batch operation modes).
Figure 1: An Xcellerex bioreactor used in an intensified perfusion process with ATF System equipment ()
From Research to Manufacturing — the Scale-Up Challenge: For companies requiring increased protein production in preclinical work, many perfusion technologies can quickly deliver. One common approach is to choose a small-scale cell-retention device that offers a high degree of confidence for scaling to a commercial manufacturing process. Scaling up a bioreactor introduces its own issues, so engineers don’t want perfusion equipment to add further complications. Several technologies have been used at large scale, and each system brings its own limitations. For example, well-known spin-filter technology, previously discussed, uses a two-dimensional screen to retain the cells. Limitations of the system (whether internal or external) arise during scale up and at elevated cell concentrations when rapid feed rates are required. Consequently, to reduce risks of screen blockage, the process duration must be shortened or the culture maintained at low cell concentration to prevent excessive accumulation of cell debris on the screen. The latter is usually what occurs.
Different but familiar problems occur with inclined or gravimetric settlers. Cells spend significant time in an external, suboptimal environment within the settler (particularly) as the size of a system is increased. Additionally, as a system is increased, when greater perfusion rates are required, raising recirculation flow rates can lead to inefficient cell separation and significant cell loss, which lowers output and increases costs.
Centrifuges have been scaled up successfully for several perfusion processes, often to very high flow rates. However, the high level of fine-tuning required to maintain the reproduceability of such systems — particularly during scale up — as well as their cost greatly discourage their use.
Despite those issues, each cell-r
etention device has a solid following among a number of companies. Skilled and experienced individuals maintain such systems. They assess and improve scale-up and scale-down performance.
For companies that require simpler systems that can be operated by a nonspecialist or that do not want to devote years to building those requisite skills, the ATF system can provide a robustly scalable process platform for most cell lines. Laboratory-scale devices are run as standard to produce the same conditions and flows that commercial scale devices will use. Two key parameters to keep constant are the filtrate flow ratio and the flow through each individual hollow fiber. Other parameters that would normally require attention — e.g., filter surface area and residence time — are factored into the equipment configuration design to limit variability potential. Scale-up is therefore straightforward to help teams build their confidence and experience rapidly. Additionally, unlike the older systems, a failure in the ATF system does not mean failure of the run. The perfusion device can be easily exchanged with another in a sterile way to continue the process. Bioreactor issues actually come to the fore: Can a large-scale bioreactor handle the oxygen demands of a cell concentration that is about 10 times higher than usual?
A Factory of the (Near) Future
A stable cell line is a prerequisite for a perfusion process if it is intended to produce a high-quality product for an extended time. Considering the state of biological manufacturing today and industry trends of the past two decades, some features of the factory of the future can be anticipated:
A Continued Move Toward Single Use: Innovations in disposable bioreactor designs have moved the industry toward their increased use. That trend is reflected by the large number of companies that are currently supplying single use Bioreactors (SUBs). Innovative SUBs from sub-one liter to 2,000 L are readily available today. Along with SUBs, significant improvements have been made in processing equipment, sensors, and other components, all with disposability in mind.
A Shortened Bioreactor Train: The ability to generate high-cell-concentration cultures combined with the ability to freeze large volumes of such cultures has made it possible to create high-volume cell banks. A single sample can be used to inoculate a relatively large bioreactor directly, eliminating multiple steps, saving time, and greatly increasing reliability.
Simplified Product Stream: Generating a filtered product stream by filtration perfusion can shorten the steps between vessel and column.
Concentrated Fed-Batch: In a process that can be considered a form of perfusion, the culture is perfused to generate ultrahigh cell concentration, greater than 108 cells/mL; and the product is also retained in the vessel. Product concentrations greater than 25 g/L have been reported. The trend to higher product concentrations is not abating.
Concentrated Perfusion: Although 1 g/L/day is routinely achievable today using concentrated perfusion, 3 g/L/day has been reported, and 5 g/L/day can be regarded as the next step. The volumetric productivity of concentrated perfusion means that at 5 g/L/day, one 500-L reactor would produce 2.5 kg of protein every day, and potentially 500 kg/year.
If these goals are achieved in the foreseeable future, there is little reason for even a high-dose blockbuster to be manufactured in anything larger than a 500-L vessel, whereas most other products could be handled with current laboratory-scale equipment. The future size of the factory, for upstream processes at least, looks very small indeed.
About the Author
Author Details
Corresponding author John Bonham-Carter is vice president of sales and business development, and Jerry Shevitz is president and director of R&D at Refine Technology, 26 Chapin Road, Unit 1107, PO Box 691, Pine Brook, NJ 07058; 1-732-993-3003, 46-76-256-5114; [email protected]; [email protected]
REFERENCES
1.) Langer, ES. 2011. Perfusion Bioreactors Are Making a Comeback, but Industry Misperceptions Persist. BioProcess J. 9:49-52.
2.) Voisard, D. 2003. Potential of Cell Retention Techniques for Large-Scale High-Density Perfusion Culture of Suspended Mammalian Cells. Biotechnol. Bioeng. 82:751-765.
3.) Shevitz, J Mizrahi, A.. 1989.Stirred Tank Perfusion Reactors for Cell Propogation and Monoclonal Antibody ProductionAdvances in Biotechnological Processes, Alan R. Liss., Inc., New York:81-1.
4.) Carstens, J. 2009. Perfusion! Jeopardy or the Ultimate Advantage?. BioProcess Int webinar.
5.) Bonham-Carter, J. 2010. Which Process Option Is Right for Me?. Bioresearch Online.
6.) Yuan, H. 2009.Cell Culture Process Scale-Up, Technology Transfer, and Assessment of Process Comparability Using Multivariate Analysis: A Case Study, BioProcess International Conference and Exhibition, Rayleigh.
7.) Alahari, A. 2009. Implementation of Cost-Reduction Strategies for HuMAb Manufacturing Processes. BioProcess Int. 7:s48-s54.
8.) Tezare, R. 2010.BioProcess International Conference and ExhibitionRunning Inoculum Cultures in Perfusion Mode to Enable Higher Seeding Densities of Production Cultures, Providence.
9.) Compton, B. 2007. Use of Perfusion Technology on the Rise. Gen. Eng. News 27.
10.) De Vocht, M. 2008.Intensification of a PER.C6®-Based Recombinant Ad35 Manufacturing Process to Prepare for Commercial Scale Production Fifth Annual Optimising Biomanufacturing Processes.
11.) Noe, W. 2009.Paradigm Changes and Technology Gaps in the Biopharmaceutical IndustryBioProcess International Conference and Exhibition, Raleigh.
12.) Yuan, H.A 2011.Platform for Late-Stage Monoclonal Antibody Process Development: Robust High Seeding Density CHO Cell Culture Process Utilizing Perfusion at N-1 StageIBC Antibody Development & Production, Bellevue.
13.) Muller, D. 2011.ATF Perfusion-Based Production Platforms for BiopharmaceuticalsESACT Conference, Vienna.
14.) Li, L. 2009. A Single-Use, Scalable Perfusion Bioreactor System. BioProcess Int. 7:46-54.
15.) Lim, J. 2011. An Economic Comparison of Three Cell Culture Techniques. BioPharm Int. 24:54-60.
16.) Whitford, WG. 2010. Single-Use Systems As Principal Components in Bioproduction. BioProcess Int. 8:34-42.
17.) Whitford, WG, and JJS Cadwell. 2009. Interest in Hollow-Fiber Perfusion Bioreactors Is Growing. BioProcess Int. 7:54-64.