A Comprehensive Solution
Innovative Viresolve Pro Family: Robust, productive, next generation
Quality and Manufacturing Expertise: Over 15 years of virus filter manufacturing experience; state-of-the-art membrane plant; comprehensive validation; raising the standard of performance and integrity tests
Industry-Leading Service and Support: Process development, optimization, and scale-up; leading the industry with spiking strategy and validation; services covering every stage of adoption
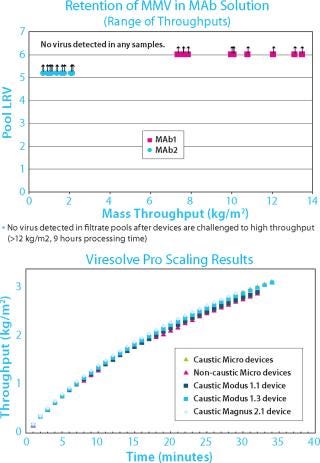
Building Assurance Test By Test (Figure 1)
No one does more than Millipore to help assure virus filter performance and compliance: end-user validation and testing; device 100% tests (newly patented binary gas test); device release tests; membrane release tests; and process and product validation.
Figure 1: ()
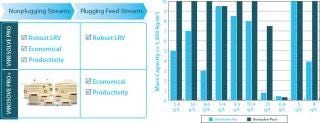
Key Benefits (Figure 2)
Process Robustness: Enables increased process robustness; protects against variability with respect to changes in solution conditions; relatively insensitive to stream plugging characteristics; limits the optimization required (superior template fit)
Figure 2: ()
Productivity: Enables high productivity, enhancing Viresolve Pro performance; increased mass capacities coupled with high protein flux; easy to install, test, and use; caustic stable; disposable flow path at all scales
About the Author
Author Details
Stephanie McGary and Janice A. Lonardo are product managers at Millipore Corporation, 290 Concord Road, Billerica, MA 01821; 1-800-645-5476; www.millipore.com.