Influenza virus vaccines have traditionally been produced by infection of fertilized hen eggs. This labor-intensive approach requires large facilities, which has led to the development of large-scale mammalian cell culture methods for future virus vaccine production processes.
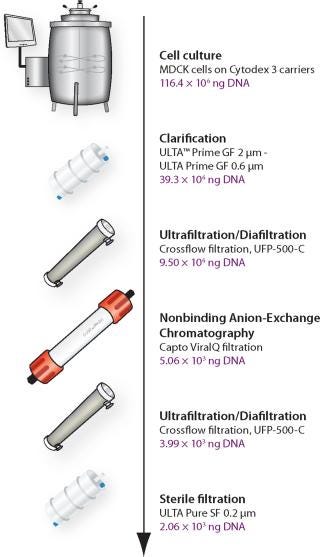
The main focus of these studies was to use generic, established, and scalable techniques for industrial production of live influenza vaccines (Figure 1) and potentially also for other viruses independent of type or size. Development of three different focus areas in live influenza processing are described: Optimization of a disposable cell culture step; a downstream purification process for efficient removal of genomic DNA (gDNA) from live influenza virus cultured in Madine-Darby Canine Kidney (MDCK) cells; and development of a fast, accurate live influenza assay.
Figure 1: ()
Cell Culture Development
WAVE Bioreactor systems were originally designed for culturing cells in suspension. However, several cell types used for cell therapy and vaccine production are anchorage-dependent and require a surface to grow on. In this study, Cytodex microcarriers were used in a WAVE Bioreactor™ to expand MDCK and Vero cells. Different mixing conditions were tested for both cell lines in order to optimize cell attachment.
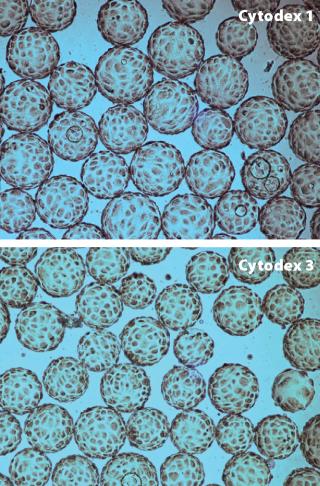
We found that composition of the cell culture medium had a significant effect on cell attachment; for example, MDCK and Vero cells cultured in low serum-containing medium attached to the microcarriers with intermittent and continuous mixing. However, under serum-free conditions, high attachment of Vero cells could be obtained only by using intermittent mixing. MDCK cells were grown on Cytodex 1 and Cytodex 3 microcarriers (Figure 2) in a WAVE Bioreactor system at working volumes of 2 L and 10 L. The cells reached a maximum concentration of 3 × 106 cells/ML. Vero cells were cultivated on Cytodex 1 and Cytodex 3 carriers at a working volume of 2 L and reached a maximum concentration of 1.4 × 106 cells/ml — the same concentration of Vero cells was obtained with experiments using spinner flasks.
Figure 2: ()
The data show that the WAVE Bioreactor system is a versatile platform for microcarrier cultivation of MDCK and Vero cells, and it is also a fast and convenient alternative to conventional stirred-tank bioreactors or roller-bottle systems.
Conclusions
Optimal microcarrier conditions in WAVE Bioreactor were developed demonstrating the potential for fully disposable influenza vaccine manufacturing from adherent MDCK cells. A generic and efficient purification process was developed for the removal of gDNA and host cell–derived impurities from MDCK cell-based influenza. A further purification step is, however, required to achieve vaccine purity according to WHO recommendations. A Biacore based influenza analysis was developed and shown to be more accurate and faster than SRID.
About the Author
Author Details
Björn Lundgren is the vaccine marketing manager at GE Healthcare Bio-Sciences AB, Björkgatan 30, 75184 Uppsala, Sweden, [email protected]; www.gelifesciences.com/bioprocess.