
This application note describes a case study of the correct sizing for a filter involved in an automated final bulk-fill system for a biopharmaceutical product. It demonstrates how customer batch-processing requirements can be attained with a robust experimental design.
Scale-Up Methodology
Figure 1 shows a process for successful scale-up. Once the desired process has been defined, filter performance can be determined by either constant flow or constant pressure testing on disc formats.
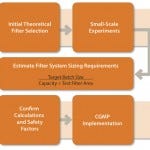
FIGURE 1: A process for successful scale-up
The data can be scaled-up easily to determine the required system size for the target process because capacity is directly proportional to filter area. Pilot-scale or full engineering trials can be used to confirm initial findings because experimental variability can be introduced through differing filter formats, drug product batches, or process equipment.
Sizing should be performed using a scale model of the target process. Ideally, processing time should remain constant, and the flow rate should be adjusted as a function of test batch volume.
The SciLog FilterTec system measures differential pressure across a filter during testing, allowing real-time assessment of filter blocking characteristics and further optimization through the use of prefiltration stages. Constant flow testing is performed until the differential pressure across the filter reaches a defined, predetermined end point.
Scale-Up Testing
To match the requirements of the process, sterilizing filtration of a high-potency drug was required. The process was defined by the maximum batch size of 25 L of bulk product and an upstream pressure limit of 2 barg.
Mid-batch filter blockage or damage to the single-use manifold resulting from overpressure was not acceptable because of the hazardous nature of the drug product.
Disc testing was performed on Parker domnick hunter’s PROPOR HC at a target flow rate of 10 mL/min, with a pressure limit of 1.6 barg, which gave a 20% safety factor. This established a capacity of 92.4 mL of solution for a 47-mm PROPOR HC disc at an actual flow rate of 8.5 mL/min as shown in Figure 2. Scaled-up relative to effective filtration area, this is equivalent to 35 L of bulk product through a 10-inch filter capsule at a flow rate of about 3.2 L/min. Therefore, a 10-inch filter capsule is capable of processing a 25-L batch while incorporating a 40% safety factor in capacity.
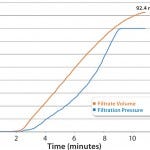
FIGURE 2: Filtrate volume and pressure over time
Confirmation of this analysis through the use of a pilot-scale trial provided further assurance that the results are repeatable on a larger scale and that the batch can be processed without risk of filter blockage.
Conclusion
Using a structured analytical approach under simulated process conditions, it is possible to predict filter performance to a significant level of accuracy at an early stage, minimizing the risk during scale-up. Use of automation during this process allows increased accuracy through the removal of potential operator variability.
Andrew Kelly is product manager at Parker domnick hunter, Durham Road, Birtley, Co Durham, DH3 2SF, UK; 44-191-410-5121, [email protected]; www.parker.com/dhsingleuse










