The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plasticequipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and regulators to safeguard the quality of drugs produced with single-use technology (SUT).
(WWW.SHUTTERSTOCK.COM)
Part 2 of this series continues the focus on cell and gene therapy (CGT) manufacturing by providing a regulatory overview. It is based largely on experience gathered from the use of these products in blood processing and biologics manufacturing. Differences between those areas and cell therapies are highlighted throughout. (Part 1)
REGULATORY CLASSIFICATION
From bone-marrow transplants to cordblood processing for therapeutic purposes, cell, gene-modified cell, and gene therapies have advanced into industrial-level production and delivery. Equipment systems used for cell and gene manufacturing are critical to ensuring that therapeutic products are produced and delivered to regulatory standards. Because of their original use in processing bone marrow and cord blood for transplantation, such equipment systems have been classified as medical devices for their clinical utility. However, with cell and gene therapeutic products becoming regulated as biological drugs, equipment systems may no longer be regulated as medical devices, but rather as manufacturing equipment. When classified as manufacturing equipment, such systems are not required for premarket clearance or approval by health authorities for commercialization (unlike medical devices). However, manufacturing equipment still is controlled through different regulations (e.g., regulations 21 CFR 211 and ICH Q7A(v) in the United States) (1, 2). Those regulatory requirements are an integral part of current good manufacturing practice (CGMP) to ensure a drug product’s quality, as referenced below:
The regulations set forth in this part and in parts 211, 225, and 226 of this chapter contain the minimum current good manufacturing practice for methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packing, or holding of a drug to assure that such drug meets the requirements of the act as to safety, and has the identity and strength and meets the quality and purity characteristics that it purports or is represented to possess (3).
Likewise, equipment systems need to perform to specifications to deliver on the critical quality attributes (CQAs) of drug products. Hence, when major manufacturing equipment is changed in an established drug manufacturing process, a predefined comparability assessment must be conducted, and new equipment units must demonstrate that they can produce comparable products. A risk-based approach to determining the effect of the change to CQAs also will determine the actions required to support the change.
Because drug products are the entity subject for regulatory review and approval for commercialization, regulatory requirements on manufacturing equipment are enforced onto drug developers and manufacturers (on equipment users, but not on equipment suppliers). However, equipment suppliers must supply good quality equipment with adequate information to enable end-users to meet regulatory requirements.
COMPENDIAL TESTS
Biocompatibility: The function of a manufacturing system is to enable production of a therapeutic agent that is safe and efficacious. Compatibility of cells with the materials used in manufacturing and storage systems needs to be assessed to ensure that the materials that constitute a manufacturing system do not negatively affect the growth or function of the cells. Although currently there is no specific guidance or standard for single-use manufacturing systems used in bioprocessing or cell therapies, the industry has adopted standards for medical devices to assess the biological compatibility of plastic materials. The International Organization for Standardization (ISO) and the United States Pharmacopeial Convention (USP) have developed similar methods to assess biocompatibility of medical devices and polymeric materials intended for making containers, parenteral preparations, implants, and other systems. USP standards for evaluating biological reactivity include USP <87> Biological Reactivity Tests, In Vitro and USP <88> Biological Reactivity Tests, In Vivo (4, 5). Those tests are designed for application to plastics and other polymers in the conditions under which they are to be used. If a material is to be exposed to cleansing or sterilization processes before its end use, then the tests are to be conducted on a sample prepared from a specimen preconditioned by the same processing.
USP <87> is used to test for reactivity of mammalian cell lines to elastomeric plastics and other polymeric materials with direct or indirect patient contact, or of specific extracts prepared from the materials under test. ISO 10993-5 is the corresponding test for determining cytotoxicity of materials (6).
USP <88> is designed for determining the biological response of animals to elastomerics, plastics, and other polymeric material with direct or indirect patient contact, or by the injection of specific extracts prepared from the material under test. Three tests are described. The systemic injection test and the intracutaneous test are used for elastomeric materials, especially elastomeric closures for which the appropriate biological reactivity tests, in vitro (USP <87>), have indicated significant biological reactivity. These two tests are used for plastics and other polymers, in addition to a third test, the implantation test, for testing the suitability of those materials. A plastic class designation (Class I–VI) can be determined based on the response of animals in the prescribed tests, with Class VI indicating that the material is not reactive in any test.
The ISO 10993 series describes the tests included in USP <88> in three separate standards: ISO 10993-6 Tests for Local Effects After Implantation, ISO 10993-10 Tests for Irritation and Skin Sensitization, and ISO 10993-11 Tests for Systemic Toxicity (7–9). ISO 10993-12 describes sample preparation and reference materials (10). The ISO, USP, or alternative qualified methods can be used to assess biocompatibility of manufacturing systems. Although there appears to be a trend in the industry to perform only USP <88> because it is perceived to be a more extensive test, it may be advantageous to perform USP <87> when SUT is used for cell therapies because the cells are the product. It also may be beneficial for cell therapy developers to perform testing on the cell types used in their products.
According to USP <1031> Biocompatibility Materials in Drug Containers, Medical Devices and Implants (11), a comprehensive biocompatibility guidance document, extracted polymers do not alter stability of products or exhibit toxicity.
Particulates continue to represent a distinct challenge to companies working with CGTs. To date, limited to no guidance for particulate control specific to CGTs is available. Because of the emerging status of CGTs, guidance documents have yet to be established. This has led the CGT industry to borrow and work with what exists for adjacent markets. Currently, the most commonly observed guidance documents followed by CGT sponsors and suppliers include USP <1> Injections, USP <788> Particulate Matter in Injections, and USP <790> Visible Particulates in Injections in the United States, along with the relevant European Pharmacopoeia (EP) and Japanese Pharmacopoeia (JP) requirements for Europe and Japan, respectively (12–14).
USP <788> has been official for several years. It defines two methods for counting subvisible particles and sets limits for containers based on size. For visible particulates, USP <1> and USP <790> offer some guidance to the industry. USP <1> sets the requirement that every final container is to be inspected for particulates to the extent possible, and containers with observable particulate matter are rejected. USP <790> further establishes reference inspection conditions and provides quantitative limits based on acceptance sampling to meet the expectation that every lot is to be essentially free from visible particulates. These guidance chapters are based mainly on the premise or assumption that the final products are clear and transparent. Obvious challenges are associated with CGT products: Most will be opaque because they contain cells as a significant part of the final products.
Further complicating the matter, a final clearance or filtration step commonly incorporated in bioprocessing and pharmaceutical industries is not possible for CGT products because the filters intended for particulate capture also would capture the cells. If a foreign particulate is observed in a CGT product, this can have catastrophic implications for patients. Discarding the lot, which for autologous products often equals a single product, is not desirable but may be unavoidable depending on the nature and potential impact of the foreign particulate on the patient. Therefore, while we continue to extrapolate information from other industries and reference their respective guidance documents for control of particulate matter, development of guidance documents specific to CGTs will be required.
It is critical to the success of CGT products in development that they be both safe and efficacious when used to treat a specific indication in relevant patient populations. Particulate risks related to a product’s final formulation are important for many different reasons. The most obvious risk is the potential for adverse events from the presence of particulates that occurs after a product is administered to a patient. The second risk is the effect of particulates on product quality itself, both during production and in a product’s final formulation. Particulates discovered in final products also increase their risk of being recalled, leading to potential clinical trial delays or failure to maintain commercial inventory. The most commonly associated risks of particulates to patients center on the threat of an immediate blood vessel occlusion and possible immunological response to foreign contaminants.
Despite the different sources and composition of particulates, there are several common types of pathogenic mechanisms for potential harm to patients. These mechanisms include inflammation from infections caused by viable organisms; inflammatory responses caused directly or through associated leachates that trigger direct tissue injury, normal and abnormal immune responses to cellular debris; and tissue damage from thromboembolisms.
With CGT products, risk to a product also is critical. The effect of inert particulates on cells and cell cultures will differ greatly depending on the properties of the particulates and cell lines. Cellular adhesion can be affected by exposure to particulates, depending on their basic topography and whether they are taken up by cells. Particulates also can be detrimental to the viability and functionality of a cell culture. Leachables and extractables from such particulates can alter an environment’s pH or produce compounds that are toxic to cells — both immediately and over time — which could affect product stability directly.
MATERIAL TESTS
USP <661> Plastic Packaging Systems and Their Materials of Construction (15) refers to a set of analytical standards to help ensure the safety of health-related products made of and/or packaged in plastic containers. They include pharmaceuticals, biologics, dietary supplements, and devices. Polymers outlined in USP <661> subchapters include high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP), polyethylene terephthalate (PET), polyethylene terephthalate G (PETG), and plasticized polyvinyl chloride (PVC). For plastic packaging to be approved for use with an FDA-approved therapeutic product, data must show that the material/packaging conforms to USP <661> standards and performance criteria.
USP <665> Polymeric Components and Systems Used in the Manufacturing of Drug Products (16) is a new general chapter that addresses the qualification of polymeric components used in the manufacture of both pharmaceutical and biopharmaceutical active pharmaceutical ingredients (APIs) and drug products. USP<665> has been proposed and published for public comment, but as of our publication date, has not been adopted and is currently under review with the USP.
STORAGE OF SINGLE-USE, BAG-BASED SYSTEMS
Bags should be transported and stored in their original carton packaging (protected from light) in a protected warehouse and used before their expiration date. Recommended storage conditions are at ambient temperature (between +5 °C and 40 °C) and a relative humidity of <85%.
Although single-use bags are tested at the bag manufacturer’s facility, improper handling can cause problems. When dealing with highly valuable patient samples, testing at the point of use may be advisable. Leak detection typically is tested using sterile air.
SUT STERILIZATION VALIDATION AND STANDARDS
Cell therapy manufacturing shares much in common with general pharmaceutical manufacturing requirements, and that knowledge base does a good job of setting standards for how you should be testing your SUT products. The sterility standards that apply for SUT in cell therapy manufacturing (Table 1) are the same as those that apply for other classes of biopharmaceutical manufacturing.
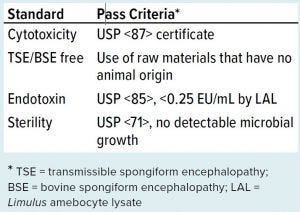
Table 1: Biocompatibility and sterility standards for single-use technologies in cell therapy manufacturing
As mentioned above, cytotoxicity is always a concern, and ensuring that the problem is not the disposable containers in contact with your cells is paramount. USP <87> provides guidance to minimize that risk (4).
Bovine spongiform encephalopathy (BSE) is a form of transmissible spongiform encephalopathy (TSE) and a prion disease that affects the brain and nervous system. To reduce the chance of contamination, it is best to minimize or avoid use of animal-derived raw materials. That can be achieved in three ways: using products that are not of animal origin (e.g., using vegetablederived stearates rather than bovine (tallow) derived); certifying that animalderived raw materials are from a source that is known to be BSE/TSE free; or ensuring that animal-derived raw materials or components that must be used meet or exceed requirements specified in the Note for Guidance on minimizing the risk of transmitting animal spongiform encephalopathy agents (17).
Endotoxins can cause fevers and other symptoms in humans who are exposed to them. These toxins come from Gram-negative bacteria walls that contain lipopolysaccharides (LPS). Typical testing for that is conducted with a Limulus amebocyte lysate (LAL) test, with USP requiring pooled testing of the production lot. USP <71> Sterility Testing describes the sterility testing protocol (18). Depending on the size, shape, and application of a product, the sterility test can be performed by using the membrane filtration method, the direct transfer method, or the product flush method. Many SUTs are closed chambers with ingress/egress only through small-bore tubing. Therefore, product flush followed by membrane filtration and incubation in soybean casein digest medium/fluid thioglycollate medium (SCDM/FTM) are commonly used, which differs from the application of USP <71> for therapeutic products or medical devices and is not relevant to the current lot sizes produced for most cell therapy manufacturing (<1,000 units/lot).
USP <1211> Sterilization and Sterility Assurance (19) supports USP <71> and describes the sterility testing environment with which CGT manufacturers should comply in validating sterility of their products.
SUT QUALIFICATION AND ACCOMPANYING DOCUMENTATION FOR EQUIPMENT
Equipment systems must be labeled appropriately for their intended use. With heightened requirements on traceability and prevention of mix-ups for CGT products, labeling and special coding on SUT can enable chain of custody and traceability. An integrated traceability approach such as this also can apply to ancillary materials and other manufacturing components to enable identification of defects and causes of failures as well as for security management throughout a supply chain.
Equipment systems are supplied with accompanying documentation, such as instructions for use, declaration of conformity, and so on. Suppliers also provide testing data such as extractable and leachable data, data on particulates, and often performance data to support installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). When proprietary data are associated with equipment systems, including SUT, suppliers can prepare a drug master file (DMF) for end users to cross reference in their regulatory applications. As a best practice, a regulatory support file containing all essential information should be provided to users to facilitate regulatory inspection. Such information on file also will help users compile equipment information in their regulatory applications.
LOOKING AHEAD
Part 3 will conclude this series with a discussion on best practices for supplier selection, qualification, and validation to ensure supply chain security.
REFERENCE
1 21 CFR 211: Current Good Manufacturing Practice for Finished Pharmaceuticals. US Fed. Register 78, 2013: 4307–4323.
2 ICH Q7A(v): Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients. US Fed Register 66(186) 2001: 49028–49029.
3 21 CFR 210.1: Status of Current Good Manufacturing Regulations. Current Good Manufacturing Practice in Manufacturing, Processing, or Holding of Drugs, General. US Fed. Register 74, 2009: 65431.
4 USP <87> Biological Reactivity Tests, In Vitro. US Pharmacopeia–National Formulary. US Pharmacopeial Convention: North Bethesda, MD.
5 USP <88> Biological Reactivity Tests, In Vivo. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
6 ISO 10993-5 C Biological Evaluation of Medical Devices, Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
7 ISO 10993-6: Biological Evaluation of Medical Devices, Tests for Local Effects After Implantation. International Organization for Standardization: Geneva, Switzerland, 2016.
8 ISO 10993-10: Biological Evaluation of Medical Devices, Tests for Irritation and Skin Sensitization. International Organization for Standardization: Geneva, Switzerland, 2010.
9 ISO 10993-11: Biological Evaluation of Medical Devices, Tests for Systemic Toxicity. International Organization for Standardization: Geneva, Switzerland, 2017.
10 ISO 10993-12: Biological Evaluation of Medical Devices, Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
11 USP <1031> Biocompatibility Materials in Drug Containers, Medical Devices and Implants. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
12 USP <1> Injections. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
13 USP <788> Particulate Matter in Injections. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
14 USP <790> Visible Particulates. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
15 USP <661> Plastic Packaging Systems and Their Materials of Construction. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
16 USP <665> Polymeric Components and Systems Used in the Manufacturing of Drug Products. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
17 EMA/410/01 Rev. 3. Minimising the Risk of Transmitting Animal Spongiform Encephalopathy Agents via Human and Veterinary Medicinal Products. European Medicines Agency, London, UK, 2011.
18 USP <71> Sterility Testing. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
19 USP <1211> Sterilization and Sterility Assurance. USP–NF. US Pharmacopeial Convention: North Bethesda, MD.
For more information, contact Kevin Ott (ottk@ socma.com). This article is published in extended form with permission from Bio-Process Systems Alliance (BPSA), 1400 Crystal Drive Arlington, VA 22202; www.bpsalliance.org. The white paper is available at http://bpsalliance.org/cell-and-genetherapy-resources.









