In the past decade, single-use bioreactors gained significant importance in manufacturing processes of monoclonal antibodies (MAbs) and recombinant proteins. The success of such technologies comes from their numerous advantages over multiuse equipment (1). Overall, disposables offer an answer to some key challenges in the biopharmaceutical industry: time to market, validation complexity, process security, production efficiency, and cost of goods.
Because the same challenges apply to vaccine production, single-use bioreactors also have the potential to optimize manufacturing processes and offer further benefits to the animal vaccine industry. Vaccine manufacturers require great flexibility in their production equipment because they typically produce several different vaccines at reduced volumes using a number of cell lines. That need is particularly important to animal-health companies that manufacture not only different antigens, but also antigens for different target species. The enhanced flexibility offered by single-use bioreactors can make them valuable alternatives for such production plants.
Experts predict that future needs for animal vaccines will increase significantly (2). A number of factors influence this. Most human infections are zoonoses, having animal origins. So animal health is a concern partly because it can affect human health risks. As the world population grows, the global demand for animal protein (meat) is increasing. Also, animal health is trending toward prevention rather than treatment because of modern concerns about antibiotics and residues in meat. All of that has led to HIPRA's decision to evaluate the efficiency of single-use bioreactors for future vaccine production. Located in the northeast of Spain, HIPRA S.A. is an animal-health company that makes biological products for poultry, swine, ruminants, dogs, rabbits, and fish. Here we describe the company's experience with single-use bioreactors (SUBs) — particularly regarding the differences in working with multiuse bioreactors (MUBs) and SUBs for virus production. The goal of this study was to evaluate whether SUBs could be a viable alternative to stainless steel for antigen production in animal vaccine manufacturing.
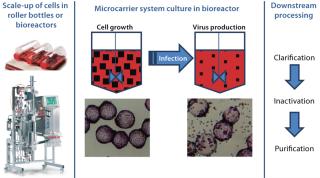
General Virus Production Process: Surface-dependent, continuous cell lines are typically used for cell-culture–based production of viruses. So for this study, commonly used cells such as Vero cells represent a broad range of different virus-production processes. Figure 1 overviews a typical process for virus production in cell culture. The process was scaled up in the SUB according to the standard process scheme.
Figure 1:
Comparing MUBs and SUBs
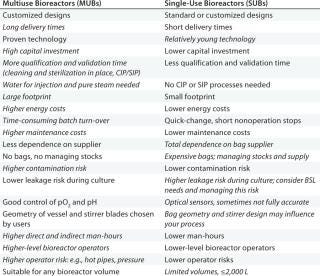
Large-scale MUBs are time-proven technologies around the world. Although they have demonstrated process efficiency, a company must consider many aspects when acquiring one. Large-scale MUBs often evolve as customized solutions. Such customized development requires a large amount of resources on both the supplier and user sides. Delivery times are very long — usually more than a year — with several months needed for qualification before a system can be used in production. Capital investment and operational costs are high, and the equipment footprint is large.
With the introduction of SUBs, alternative solutions became available for commercial cell-culture–based manufacturing. And although these offer many benefits over stainless steel, new points must be considered: e.g., stock management of consumables, waste disposal, manual operations in bag installation, and ensuring proper connections. Table 1 lists major differences between SUBs and MUBs. Clearly, the former have the potential to bring more benefits than the latter. However, details can vary from one process to the next — and depending on a facility's location.
Table 1
Table 1: 194;
Implementing SUBs: When a company considers single-use technologies, one important question must be answered first: Are you changing from multiuse or beginning directly with single-use technology?
When beginning with MUBs (Photo 1), you already have a high level of experience and knowledge in running processes with them. Your systems are well-characterized (3), enabling a good understanding of your culture's behavior in them.
Photo 1
Photo 1:
Several SUBs are different in design from stainless-steel MUBs, especially in the essentials of vessel design, gassing modules, and mixing technologies. The more those design parameters deviate from the conventional standard, the higher your need will be for process optimization and adaption when making the change to single use. Fortunately, some technology vendors offer single-use solutions that comply with conventional standards to facilitate process transfers (4).
When beginning with single-use bioreactors, by contrast, you face fewer limitations if you have smaller-scale bioreactors of similar design. In such a case, you can develop your process at small scale, then perform scale-up trials and transfer easily to your final production scale. However, it does help if your SUBs are based on a conventional design. That eases interpretation of process results, helps you anticipate mixing behavior and gassing effects, and facilitates set-up of your scale-up experiments.
When choosing a SUB manufacturer, consider the following points:
Performing actual trials with different single-use bioreactors before supplier selection allows you to challenge each technology with your specific process and cells.
Supplier reliability is essential. The more experienced the vendor is with single-use technologies (not limited to bioreactors), the better that company will understand your needs and be able to support your process. A supplier should offer appropriate production capacity because security of supply is critical when working with these technologies.
What will be your final scale? Some suppliers do not offer all scales. If you need to move to volumes larger than 1,000–2,000 L, you may need a hybrid solution with a single-use seed line and stainless-steel production bioreactors.
The supplier's validation package is important, as is its strategy to ensure a high level of batch-to-batch consistency. Variations among supplier batches could put your own process consistency at risk.
Implementing SUBs
The main issues to highlight in SUB start-up are set-up and process times, sensors, and handling of equipment.
Set-Up and Process Times: SUB installation and commissioning are easy and fast. You need only electrical power, water, and gas supplies for the system. At HIPRA, this saved about two months of set-up time. Also, because the vessel requires no sterilization or cleaning, equipment down-time can be significantly reduced. This potentially increases productivity by 15–20%, allowing more batches to be realized each year.
However, additional process transfer time needs consideration. Depending on your bioreactor selection, process parameters may need to be modified before you have a robust and similar process as was established in the previous multiuse setup. Furthermore, additional process time can be linked to emptying vessels at the end of culture processes when no sterile air pressure can be used. Overall, the time saved by using SUBs is significantly more than that of multiuse processes.
Sensors: Optical, single-use sensors supplied with many SUB bags reduce the risk of contamination and limit the time needed to prepare a vessel for its next culture (with no autoclaving of sensors required). When single-use sensors cannot be used for a given process, however, there is no need to base your bioreactor selection on this factor alone. All vendors that integrate single-use sensors offer an option of introducing “traditional” pH and DO probes (Photo 2).
Photo 2
Photo 2:
Handling of SUBs and MUBs is different in several ways. For example, aseptic connections aren't automated with the former, so connections must be made manually. Such connections are ideally realized by specific welding devices, which reduces the risk of manual errors. Sterile welding and sealing of thermoplastic tubing are key technologies that offer the highest flexibility to users who require a solution to accommodate multiple connection and disconnection cycles. So it is important to evaluate your current process set-up and exchange nonweldable tubing for suitable thermoplastic tubing wherever connections and disconnections are required.
Overall, SUBs require more manual handling than highly automated MUBs. With the more fragile polymer vessel material, it becomes important to perform proper risk analysis of handling procedures. Bag assembly and installation in a holder (Photos 3 and 4) are especially important aspects to consider. They become more complex with increasing vessel size.
Photos 3 and 4
Photos 3 and 4:
Because vaccine manufacturers work with viruses, financial risk is not the only type of risk to be considered. Biosafety risks are higher with vaccines than in recombined proteins or MAb production. SUB vendors therefore should not only be evaluated based on product performance, but also on the level of quality and robustness they offer. How can they contribute to proper biosafety risk mitigation? Such concepts are common practice in the vaccine industry, and examples of such studies specifically using single-use bioreactors can be found in the public domain (5).
During biosafety risk mitigation evaluation, vendors should be challenged in their bioreactor's means of preventing bag integrity loss. Those may include protection against sharp objects, overpressure protection, and measures against overheating. Furthermore, they should include ways to contain an environmental contamination in case of bag damage — for example, with a containment platform installed below the bioreactor.
Appropriate training and support from your selected vendor are essential to reducing risks. However, even with perfect training, we must be continually aware of risks involved in single-use. It is important to remain vigilant regarding human errors, which can lead to bag damage and consequent leaks. To mitigate the risk of damage passing undetected, HIPRA recently implemented a specific bag test developed by its selected vendor. The device (Photo 5) allows us to test bioreactor bags for leakage before use but after installation in the bag holder and after all connections are made (6). That allows us to verify that all connections are correctly made and no damage occurred during bag transport, storage, or installation. Implementation of the bag tester thus significantly reduces financial and biosafety risks.
Photo 5
Photo 5:
Process Results
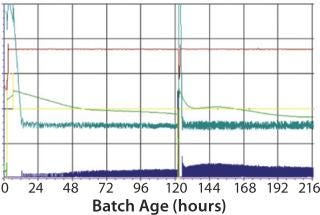
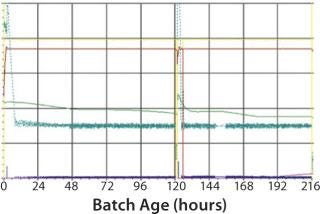
For this study, the following criteria were used for specific virus production to be evaluated: different continuous cell lines (Vero and glioma bovine kidney, GBK); the number of processes where these cells are involved; the number of batches of antigen produced per year. Cell growth in the bioreactor takes four to five days, and virus replication after infection takes from two days in some processes to four days in others. HIPRA used a 200-L, stirred, single-use bioreactor from Sartorius Stedim Biotech (BIOSTAT STR 200L) as our production bioreactor. Process results were challenged against a “gold standard” plot generated from a robust process in a multiuse bioreactor (Figure 2).
Figure 2:
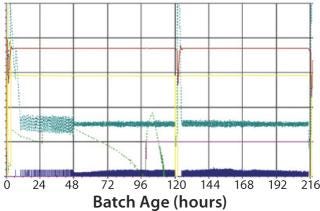
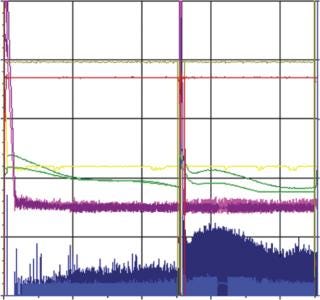
As mentioned above, moving to single use requires some time for adaptation. This is illustrated by the fact that during first use of the SUB, we observed a lower cell growth and final virus yield due to improper oxygen and pH control (Figure 3). After parameter optimization, we established a robust process control ensuring good cell growth and virus yield (Figure 4). The time for transferring from MUBs to SUBs depends on the process and cells involved. For some processes, only few runs will be required to optimize the SUB, whereas others can require more effort. From our experience, generally only a few batches are needed to make the transfer and establish a robust process.
Figure 3:
Figure 4:
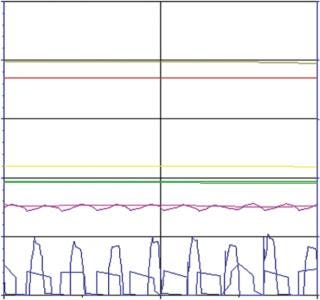
When comparing the robust SUB process against the MUB gold standard, we found a very close correlation between the corresponding parameters resulting in a similar growth profile and productivity (Figure 5). Only for the oxygen flow did we observe a different behavior due to differences in the set-up of the oxygen control between the bioreactors. In both cases, however, the pO2 set point could be perfectly maintained. Although one process required high oxygen peaks for short periods, the other bioreactor required longer but smaller peaks (Figure 6).
Figure 5: MUB: dark blue = oxygen flow, pink = pO2, dark green = pH, yellow = stirring speed, dark red = temperature SUB:
Figure 6:
Our study demonstrates that single-use technologies can offer a good alternative to multiuse equipment for commercial manufacturing of animal vaccines. We found no negative impact on growth profiles, productivity, or product quality and established a high level of batch-to-batch consistency. SUBs offer a solution for specific challenges that vaccine manufacturers face, such as the large variety of processes run in each facility and pressures on final-product cost and time to market.
As the maturity level of SUBs continues to increase and supporting technologies are introduced to market for early detection of bag damage from misuse or manual error, risks linked to the use of plastics can be reduced drastically over time. Proper biosafety risk mitigation plans can will be realized, allowing companies to move safely from MUBs to SUBs even when working in biosafety level 2 or 3 environments.
HIPRA S.A. has now moved its first processes into SUBs and successfully established manufacturing of animal viruses using these systems.
Author Details
Corresponding author Jordi Ruano Bou is biologics production director at HIPRA S.A., Avda. La Selva, 135, 17170-Amer, Girona, Spain; 34-97243-0660; [email protected]; www.hipra.com. Davy De Wilde is director of marketing fermentation technologies at Sartorius Stedim Biotech GmbH.
REFERENCES
1.) Eibl, R, C Löffelholz, and D Eibl Eibl, R and D. 2011.Single-Use Bioreactors: An OverviewSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley & Sons, Inc, New York:33-51.
2.) Flanagan, M 2013. Expert Eyes Surge in Vet Vaccine Demand. Animal Pharm 10.
3.) Marks, D Equipment Design Considerations for Large Scale Cell Culture. Cytotechnol. 42:21-33.
4.) Noack, U Eibl, R and D. 2011.Single-Use Stirred Tank Reactor BIOSTAT CultiBag STR: Characterization and ApplicationsSingle-Use Technology in Biopharmaceutical Manufacture, John Wiley & Sons, Inc, New York:226-240.
5.) Chaubard, JF. 2010. Disposable Bioreactors for Viral Vaccine Production: Challenges and Opportunities. BioPharm Int. (supplement) 2.
6.) Stering, M. 2013. Point-of-Use Disposable Bag Testing. Gen. Eng. Biotechnol. News 33:40-41.