Figure 1:
Due to potentially very dangerous flu outbreaks in recent years, the need for production of influenza vaccines in sufficient quantities has been expressed. Also, the time needed from vaccine design to the market and overall process efficiency needs to be improved. This, along with drawbacks of the traditional viral vaccine production in embryonated chicken eggs, has led to the use of reverse genetic techniques and cell-culture–based virus propagation. Because existing downstream processes of virus purification were developed primarily for egg-derived and inactivated influenza vaccines, the need for alternative, efficient, robust, and scalable purification methods has emerged.
Chromatography is quickly becoming a method of choice for virus purification and the relevance of CIM® monolithic supports was recently demonstrated for various viruses (measles, mumps, hepatitis A, adenovirus, rotavirus, herpes, various bacteriophages, and plant viruses). CIM® chromatographic supports are single-piece continuous stationary phases cast as a homogeneous column. They are characterized by a highly interconnected network of large-diameter channels (1,500 nm), outstanding dynamic binding capacity for large molecules (such as viruses and pDNA), and low pressure drops at very high flow rates.
In this work, a scalable and industrially relevant method for purification of different influenza A subtypes (H1N1, H5N1, and H3N2) and influenza B was developed for the production of intranasal influenza vaccine.
Sample Preparation
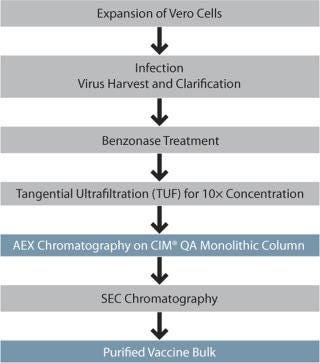
Replication deficient delNS1 influenza A and influenza B serotypes were propagated on Vero cells and expanded in cell factories. Supernatant containing virus was harvested after 36– 52 hours (subtype specific), and the clarified supernatant was treated with Benzonase. Virus was 10× concentrated by tangential ultrafiltration (TUF) using a 300-kD cut-off cassette. Figure 1 presents the overall process scheme.
Figure 1: ()
Chromatography
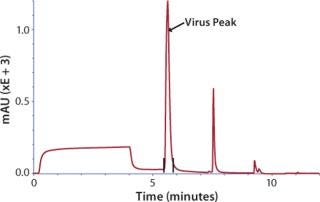
Concentrated virus was loaded on a CIM® QA (quarternary amine) disk monolithic column or an 8-mL CIM® tube monolithic column and then eluted with NaCI in HEPES buffer (Figure 2). For the polishing step, eluate from CIM® QA was loaded on size-exclusion Sepharose 6FF column, and virus was eluted in void volume.
Figure 2: ()
Table 1: Virus yields and contaminants depletion for influenza A and B purification process
Table 1: Virus yields and contaminants depletion for influenza A and B purification process ()
Results
Virus recoveries were evaluated by TCID50 and HA assays and contaminants (proteins and DNA) were determined by BradfordUltra and PicoGreen assay. Overall process virus yields are >25%, with 99.9% DNA depletion and 99% host-cell protein depletion. Dynamic binding capacities were determined to be in the range of 10.3 log10 TCID50/mL support.
Conclusion
Cell-based virus production combined with influenza virus purification process enables the production of safer, more specific, and reliable Influenza vaccine in a short period of time. CIM® monolith technology efficiently works for purification of several replication deficient deINS1 influenza virus serotypes (H1N1, H3N2, H5N1, and influenza B), and it can be performed quickly and scalably within several hours using a TUF system and standard HPLC system equipment.
REFERENCES
1.) Roethl, E. 2008.An Industrial Platform for Purifying Influenza Virus Particles. Poster presented at BioProcess IntConference and Exhibition, Anaheim.
2.)Avir Green Hills Biotechnology, Vienna.
3.)BIA Separations, Ljubljana.