
WWW.ISTOCKPHOTO.COM
The primary goal of container–closure integrity (CCI) is to maintain the sterility and product quality of parenteral biopharmaceuticals throughout their shelf life and use. Guidelines detailing the initial CCI qualification and validation requirements have been defined and can be found in the US Pharmacopeia chapter 1207 (USP<1207>) (1). The guidelines described in USP<1207> can be applied to any common CCI testing (CCIT) method to achieve a method suited for its intended use within a drug product lifecycle. CCI is not a single time-point event, but rather an integral and holistic process. It is evaluated and stressed throughout the manufacturing lifecycle of a sterile drug product (e.g., during primary package development, line qualifications, product manufacturing qualifications, stability testing, change-control process, and shipping studies) and tested when required.
Within the pharmaceutical industry, the CCIT technology landscape is changing rapidly, and new technologies are emerging to create a wide range of testing methods and applications. However, is the availability of new technologies by itself a sufficient reason to implement them across all stages of a drug product lifecycle? In other words, if a new CCIT technology doesn’t improve the assurance of product quality/safety significantly, isn’t fast enough for most fill lines, or won’t with many containers and devices, what would be the advantage to implementing such technology?
There are benefits to the use and implementation of new and more sensitive technologies as appropriate, but as a new technology is developed, should it influence regulatory guidance and expectations of the industry? In the end, the objective of CCIT should be to demonstrate the integrity of a container–closure system (CCS), assuring product quality and patient safety. The objective should not be to demonstrate the ability to resolve an unnecessarily tight specification simply because a method has that capability.
Because many technologies can be applied to CCI testing, it is important to understand that no single, universal test method is appropriate or practical for all leak test applications within a CCI lifecycle and for all products and presentations (2). Although many companies represented in the BioPhorum Operations Group (BioPhorum) fill–finish CCIT workstream currently have experience with new and/or more sensitive technologies, some practical, technical, and regulatory hurdles have prevented implementation of those technologies. Ingress methods still are used widely and accepted broadly across the industry for CCIT across almost all stages of a drug product lifecycle. Most marketed products were developed or manufactured with dye ingress as the CCIT method of choice, and those marketed products remain safe to use.
Dye ingress (liquid tracer test) is still the most widely used CCIT method across the biopharmaceutical industry (3). As a physical limit test method that’s part of a well-defined and executed holistic approach to CCI, dye ingress testing can provide equal product quality and safety assurance for package-seal integrity with respect to microbial contamination, compared with the application of deterministic methods (1). Here we provide guidelines and best practices for the qualification and/or validation and use of a dye ingress test method within a company’s holistic approach to CCI.
Purpose
Our focus here is not on the creation of a new method or approach to ingress testing, but rather to demonstrate that ingress methods remain an important and necessary part of demonstrating CCI across the biopharmaceutical industry. Ingress methods have shown that they can demonstrate readily the required sensitivity and robustness needed in our industry today. Such methods still are needed for devices and a number of other products with which other physical CCI methods are not a practical option. Some factors that support the industry’s ongoing continued application of ingress methods are detailed below.
Products: Some products do not require technologically sophisticated approaches to demonstrate integrity of their CCSs. Some product types can cause issues with specific methods — e.g., powders or proteins can block potential breeches in a CCS, which often still can be detected by dye ingress methods. Requiring that all packages be absolutely free from gas leakage is not necessary or practical. Rather, the significance of leakage rate in relation to product quality, storage, and usage conditions must be considered. Leakage into and out of intact packages should be such that there are no adverse effects on a specific product’s safety or stability.
Devices: Many devices are physical combinations of a primary container (containing a drug) with a delivery device. The practicalities of applying some methods to an assembled device prevent their use from a practical perspective when compared with an ingress method. Such challenges include
Limited dead volume in the part of a device that is critical for a sensitive detection by vacuum decay
Limited or no access to a syringe or cartridge through a nonconductive device shell that prevents the use of high voltage leak detection (HVLD)
Limited visibility to headspace and/or masking effects from device parts affecting headspace analysis.
It is important to note that use of many deterministic methods is limited because they require disassembly of devices before a CCIT. In most cases, that is not possible without creating a high risk of altering the CCI of a primary container (and creating false positives as a result of removing it from an assembled device). Disassembly also can pose a risk to operators.
USP <1207>: The latest chapter of informational guidance on CCIT from USP implies that ingress methods are “not preferred.” The BioPhorum team believes — based on many years of experience and experimentation — that this implication unfairly depicts ingress methods. In 2017, we published our response to the publication of the revised USP<1207> in BioProcess International (4).
Cross-Industry Survey Data and Widespread Use: BioPhorum has conducted a cross-industry survey of CCIT methods currently applied across product lifecycles (unpublished data at time of writing; results of the survey to be published). The survey comprises data from 11 of the industry’s leading biopharmaceutical manufacturers and considers the five most common biopharmaceutical product presentations. Results show that dye ingress methods are overwhelmingly the most commonly applied CCIT method across the biopharmaceutical industry and across all product types.
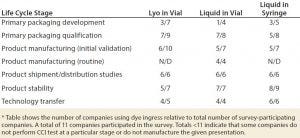
Table 1: Current use of dye ingress CCIT methods within the biotechnology industry for selected product presentations* (N/D = not done)
Looking toward the future, industry CCIT subject matter experts (SMEs) from most companies still would choose to apply dye ingress methods at specific stages of the lifecycles of their different products in the near future, despite the availability of deterministic methods. The exceptions were those respondents whose parent companies had already invested in a platform (Table 1).
When and When Not to Use the Dye Ingress Method
Overview of the Method: A CCS is immersed in a colored dye solution contained in a pressure vessel. Samples then are exposed over specified time intervals to predefined vacuum and/or pressure challenge conditions. After exposure to dye ingress challenge conditions and washing, the CCS is evaluated by comparing the content of the containers against positive and negative dye controls for dye penetration. Comparison with controls is performed either visually using the same type of containers or by spectroscopic measurement at absorbance maximum of the dye.
Advantages of Test Method — When to Use: Dye ingress is a straightforward test. Liquid ingress is the primary and practical mode of microbial ingress. So dye (liquid) ingress is a precursor for microbial ingress and thus a conservative surrogate test. The dye ingress test is a universal method with respect to the test article type and provides several benefits.
Different product presentations (vials, syringes, devices) can be tested with positive leakage controls and other assay controls.
The dye ingress method is versatile, so there are no limitations on product conductivity, headspace composition and pressure, or the nature of a product as a liquid or lyophile.
The dye ingress method requires no sophisticated equipment, only a chamber and a time-dependent pressure control (vacuum–pressure cycles), and it allows for simultaneous testing of numerous and different types of samples.
The method’s sensitivity (breach size) is appropriate for routine use; e.g., a 20-µm pinhole can be detected reliably in widely used drug product CCSs.
Direct correlation to microbial CCI (mCCI) — potentially under the same conditions — is possible.
Fully assembled complex combination products can be tested, thereby facilitating shipping studies and stability testing.
Historic data usually are available at most pharmaceutical companies, providing confidence in the dye ingress method and data.
Regulatory authorities are familiar with the dye ingress test, and regulatory expectations regarding expected method sensitivity are available in the public domain.
The same method is applicable for different testing points in a product lifecycle.
Conducting an ingress method provides first indication or simulation of the behavior of containers during and after air shipment (because of the pressure–vacuum cycling used in the method).
Those benefits are the primary reasons why dye ingress is the most commonly used method to support process characterization and validation of parenteral products and as a test method to demonstrate integrity of a CCS during product stability.
Limitations and Gaps of Test Method — When Not to Use: A dye ingress method would be inappropriate when the purpose of a CCIT is to demonstrate the absence of gas ingress or egress from a CCS or to detect gas flow rates into or out of a CCS (including loss of vacuum).
Some challenges with using the method remain. Differences often are found in process parameters between company-specific methods and the dye ingress test described in the US Pharmcopeia and in the European Pharmacopoeia, which means that no “standard” method exists. The dye ingress method is a destructive test and therefore not a practical option for application in 100% testing. Suitable controls and operator training need to be in place to reduce the risk of variability on the method’s end-point (discoloration of CCS due to dye penetration).
The method generally is not as sensitive as helium-leak testing, laser head-space analysis, or certain other CCI methods. So the dye method is not recommended for CCS qualification for which a product or CCS is required to be more than liquid-tight throughout its lifecycle or investigation of integrity specific to certain parameters (e.g., loss of inert headspace and oxygen ingress).
Factors and Parameters
The specifics of a test method’s development rely on the purpose of that test, what it is intended to demonstrate, and the product and presentation to which it is being applied. Several critical factors and parameters must first be considered and understood to adequately qualify a CCIT method.
Limit of Detection (LoD): The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample that can be detected but not necessarily quantitated as an exact value (5). For an ingress test method, the LoD identifies the smallest concentration of dye that the method can observe. Typically, this test method is not quantitative, but verification and validation should demonstrate that it can detect ingress at the defect size/leak rate of interest consistently. The quantity of dye penetrating into a CCS depends on several factors (e.g., applied pressure, vacuum, and absence of a gas) that must be understood and optimized to achieve adequate method sensitivity.
The 20-µm “Standard”: The current expectation from the US regulatory agencies is that methods should be capable of detecting defect sizes equal to or below a 20-µm diameter. This defect criterion is applied to routine test methods and is an expected positive control. Use of positive controls with defects ≤20 µm is de facto standard industry practice for method validation and routine CCIT. The US Food and Drug Administration (US FDA) effectively expects confirmation that dye ingress methods can detect leaks ≤20 µm (6).
Positive controls demonstrate that a method is executed as intended, the method equipment functions properly during testing, the method has the necessary sensitivity, (e.g., detection of 20-µm diameter defects), and other method parameters are set appropriately as validated.
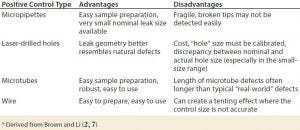
Table 2: Common artificial leaks used as positive leakage controls
A common way to prepare positive control samples is to laser-drill a container (3). Table 2 lists other methods used to create positive controls.
Method Parameters: Example parameters have been presented in a number of guidance documents (8–11). Our team also surveyed 11 leading global biopharmaceutical companies on the parameters that are applied across the industry. Clearly, results show that the bioindustry uses considerably more stringent and aggressive conditions than those outlined in the guidance documents. More important, that translates into the industry’s methods demonstrating a higher sensitivity. Those data deliberately have not been outlined here because the specific parameters used vary too widely and highly depend on the product tested and its presentation.
A number of test parameters play an important role during the performance of ingress testing and can influence the sensitivity and probability of detection of leakages.
Differential pressure conditions (vacuum and pressure); a greater pressure differential encourages traces liquid passage.
Submersion times during and after differential pressure application; longer times usually allow for greater tracer liquid passage through the leak paths.
Holding time between tracer liquid challenge and final inspection; some tracer liquids visibly fade or absorb onto package surfaces over time.
Tracer liquid surface tension and viscosity; lower viscosity and surface tension allow for detection of smaller leaks.
Other parameters play a vital role in method performance:
Tracer detection parameter and analytical methods (visual inspection or with spectroscopy), including volume of product solution; inspection environment parameters; lighting intensity and wavelength; background color viewing angle and test sample visibility; time allowed for inspection (pacing) and breaks to lessen operator fatigue; test sample content extraction procedure (wavelength, UV, or fluorescence); and comparison with negative control
Characteristics of the product liquid (e.g., protein concentration) or cake (e.g., hygroscopicity) in a test sample.
Pressures and Times: USP <381> and Ph. Eur. 3.2.9 (8, 9) cite a relative vacuum pressure of –27 kPa as an appropriate test pressure. ISO 8362-5 Annex C quotes a similar test pressure of –25 kPa. USP 31 <381> and Ph. Eur. 3.2.9 recommend application of vacuum pressure for 10 min, and ISO 8362 (10) has an application time of 30 min.
ASTM D6653:2010 (11) describes a test method using a pressure of –46.4 kPa for 60 min with a gradual pressure built up back to atmospheric pressure at a speed of max 25 mbar/min while a package system is submerged in the tracer liquid. Two additional time-dependent aspects of a tracer liquid ingress test method that should be considered are the relaxation time and the hold time between test execution and final inspection. Some tracer liquids visibly fade or adsorb/absorb onto package surfaces over time.
Tracer Liquid (Dye) Choice and Surfactant Use: For dye ingress testing, USP and the International Standards Organization (ISO) suggest a dye (e.g., methylene blue) at a concentration of 0.1% w/v (1 g/L). Different concentrations can be validated for use. Surfactants typically are added to a dye liquid to reduce its surface tension. A lower surface tension potentially enhances the detection of defects and smaller leaks because it improves the wetting properties of a package surface.
Container and Headspace Considerations: Developers of ingress leak tests distinguish two main test groups in relation to tested containers: the group of rigid containers (e.g., glass vials) and the group of semirigid containers (e.g., plastic ampules) and flexible containers (e.g., plastic bags). The displacement of the plunger for a syringe or the reversible deformation of a flexible container partly compensates for the pressure and vacuum differences, thus forcing a dye into a container system that is integral to an ingress method. Different pressure parameters are required to develop a dye ingress method with the same sensitivity as rigid containers.
Product-Related Considerations: Formulation characteristics such as viscosity, surface tension, color, absorption properties (e.g., fluorescence or UV absorbance) and clogging capabilities (e.g., at high-concentration of protein or suspensions) can influence ingress test results. The fill volume can play an important role when the dye is more or less diluted.
Detection — UV and Visual: For tracer liquids to be detectable (visible) inside a test sample, dye needs to be dissolved in the product liquid. So all inner surfaces need to be wetted, and lyophilized products need to reconstituted before detection.
Detection of a positive result from an ingress method can be determined by using either instrument-based methods (e.g., UV-absorbance measurement for dyes) or by visual examination of the degree of coloration. A container must be transparent and exterior surfaces free from labels, dirt, and residual dye for visual inspection or spectroscopic analysis (if directly through a container).
Visual inspection is the most practical and commonly used detection method for ingress testing. Some possible impact factors associated with this method are lighting (type of light, intensity, position), background color and position, sample distance to inspector, use of negative and positive dye control samples, time allowed for inspection (pacing), and breaks to lessen operator fatigue.
Analytical measurements include spectrophotometric analysis or other types of detection systems (e.g., ion analysis if salt is a tracer). When using such methods, an extraction process usually needs to be established and should be optimized to prevent contribution of tracer residues from outside surfaces of the container. A baseline determination also is required to compensate for background contribution of the product solution itself. During qualification of a spectrophotometric measurement assay, you could establish a relationship between the concentration of a dye in the product solution and the leak size to which it correlates.
How to Validate a Dye Ingress Method
General Considerations: Any CCIT method, including dye ingress, should be qualified or validated appropriately for its intended use based on available scientific knowledge. The method qualification and validation should be performed by qualified personnel using qualified equipment.
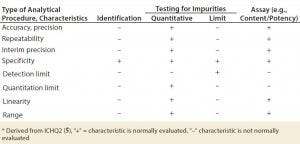
Table 3: Recommended characteristics of a limit test
Guidance on Validation: The CCIT by the dye ingress method is validated in accordance with ICH Q2 (6). The key parameters relevant to this method include its sensitivity, detection limit, and specificity. In our experience, other important factors include variability/precision and repeatability/reproducibility. Those factors are important for easing concerns regarding the probabilistic nature of an ingress method. Dye ingress testing represents a “limit test” (Table 3).
Sensitivity (“Detectable Leak Size”): The product-quality risk posed by a potential leak in a CCS is related to the ingress of microorganisms and therefore to sterility of a product.
The method must have sufficient and appropriate sensitivity to determine movement of liquids into or out of a sealed container. Such movement would be indicative of a sufficient loss of integrity and of potential microbial contamination and/or loss of product. Sensitivity in terms of leak size (“quantitation limit”) should be appropriate for the intended use of the CCIT method, with a minimum leak size of ≤20 µm (12) being consistently detected. A move toward ever-decreasing defect sizes is more relevant to gas exchange than it is to liquid ingress and should be considered in determinations of appropriate sensitivity for a method.
It is important to note that, based on what a primary container needs to maintain (e.g., sterility, protection, a critical headspace, or vacuum), dye or microbial ingress may not be the correct means of ensuring CCI. Ultimately, choosing the best method for challenging CCI should be based on individual product characteristics. Regardless of the CCI method chosen, it must be able to detect leak sizes that correspond to the leak rate appropriate for a specific product.
Specificity: A CCI method needs to demonstrate that dye penetration results in the expected coloration visually and/or spectroscopically detectable for a given drug product formulation.
Qualification/Validation of the Dye Ingress Method: Although an analytical procedure can be applied across multiple products, its performance can be affected by the product itself or by its primary packaging. The CCI procedure is validated as a limit method (pass–fail) for the determination of primary package integrity.
Validation of a CCI procedure should include the LoD for dye penetration in the specific drug product configuration. The LoD for dye penetration corresponds to the smallest level of dye added to a product that is still consistently detectable. In addition, ICH Q2 recommends validatation of the method’s precision using an appropriate number of drug product units with and without leakage (5).
Although the ingress method involves probabilities (e.g., formation of air pocket in a void), development of method parameters are such that those probabilities are not an issue in the range of the method being used. Regardless, any method (dye ingress or otherwise) must be adequately developed, qualified, and characterized.
The Right Test Method for Each Product
The most important factor of any test method is not whether a CCIT method is “preferred,” but whether it is well developed, qualified, and fit for its intended purpose. The method must be right for each specific container and product. It must suit the needs of the stage in the product’s lifecycle. The test also must be appropriate to the product and to the manufacturing process that makes it.
As we rush toward the uptake of new technologies, we must accept that older, well-understood methods still can be used for CCI testing. We agree that dye ingress or other such methods might not be appropriate for all products or presentations (for example, products that are sensitive to presence of oxygen). But deterministic methods also do not have universal applicability. Although the method is not always product or container compatible, where appropriate dye ingress continues to provide container assurance.
We agree that periodic considerations should be made with dye ingress CCIT methods as with any technology. Key questions to be answered include whether current methods provide the information and assurance required by a given container, product, and development stage. Alternative technologies should be evaluated if they are better suited for routine use and applicable in practice.
References
1 USP <1207> Package Integrity Evaluation — Sterile Products. USP 39–NF34, Supplement 1. US Pharmacopeial Convention: Rockville, MD.
2 Brown H, et al. Container Closure Integrity Testing: Practical Aspects and Approaches in the Pharmaceutical Industry. PDA J. Pharm. Sci. Technol. 71(2) 2017: 147–162.
3 Wolf H, et. al. Vacuum Decay Container/Closure Integrity Testing Technology. Part 2: Comparison to Dye Ingress Tests. PDA J Pharma Sci. Technol. 63(5) 2009: 489–498.
4 Vanness B, et al. Response to the Publication of USP <1207>. BioProcess Int. 15(1) 2017: 20-21.
5 ICH Q2 (R1): Validation of Analytical Procedures: Text and Methodology. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 1994, CPMP/ ICH/381/95.
6 Hughes PF. Well Characterized Biotechnology Products. CASSS: Washington DC, 26–28 January 2016.
7 Li L. Container Closure Integrity Testing Method Development and Validation for Prefilled Syringes. Am. Pharm. Rev. January –February (2013): 48–52.
8 USP <381> Elastomeric Closures for Injections. USP 31–NF 26. US Pharmacopeial Convention: Rockville, MD.
9 Ph. Eur 3.2.9 Rubber Closures for Containers for Aqueous Parenteral Preparations for Powders and for Freeze-Dried Powders. The European Pharmacopoeia. The
European Pharmacopoeia Commission: Strasbourg, France.
10 ISO 8362 Injection Containers and Accessories. International Organization for Standardization: Geneva, Switzerland, 2018.
11 ASTM D6653: Standard Test Methods for Determining the Effects of High Altitude on Packaging Systems by Vacuum Method. ASTM International, West Conshohocken, PA, 2001; doi: 10.1520/D6653-01.
12 Burrell LS, et al. Development of a Dye Ingress Method to Assess Container-Closure Integrity: Correlation to Microbial Ingress. PDA J. Pharm. Sci. Technol. 54(6) 2000: 449–455.
Corresponding author Scott Ewan is with BioPhorum ([email protected]). Stefanie Adler is with Janssen, Nicolas Coopmans is with GSK Vaccines, Marta Corcoran is with AstraZeneca, Sean Fadden is with Merck & Co. Inc. (Kenilworth, NJ), Chris Feeney is with Alexion, Jason Fernandez is with Biogen, Henri Hebting is with Eli Lilly, Olivia Henderson is with Amgen, Steve Klohr is with Bristol Myers-Squibb, Michael Koby is with Pfizer, Josh Lance is with Merck & Co. Inc., Brian Lee is with Pfizer, Lei Li is with Eli Lilly, Doug McNeill is with Catalent Inc., Roman Mathaes is with Lonza, Dale Moore is with AstraZeneca, Jeff One is with Abbvie, Kashyap Panya is with Regeneron, Suzanne Pellinen is with Pfizer, Amanda Scruggs is with Catalent Inc, Kamran Simani is with Shire, Mauro di Stefano is with Merck kGaA (Darmstadt, Germany), Jared Trefethan is with Bayer, Harold Van Deinse is with Shire, Brian Vanness is with Abbvie, Amy Wang is with Alexion, and Klaus Wuchner is with Janssen.











