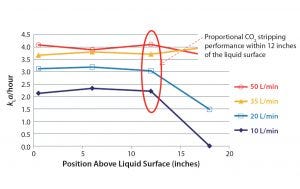
Figure 1: Crossflow sparger (with a 0.02-VVM frit) CO2 stripping performance in a 500-L SUB
Single-use technologies (SUTs) have been adopted widely in the biopharmaceutical industry for product development as well as clinical- and commercial-scale manufacturing. Over the years, suppliers of such equipment have addressed concerns about waste management, extractables and leachables, and reliability of supply — and as a result, end users have gained confidence in SUTs. Recognizing potential benefits that can be realized for both clinical and commercial operations, biomanufacturers increasingly are implementing SU solutions at larger scales in both upstream production and downstream processing of monoclonal antibodies (MAbs), other recombinant proteins, and vaccines.
The Health Advances consultancy valued the global single-use bioreactor (SUB) market at US$220 million in 2016, projecting it to grow at a compound annual growth rate (CAGR) of 21% to reach $570 million by 2021 (1). An important factor driving that growth is increase in expression titers now possible with many cell culture systems. Relatively small bioreactors (2,000 L rather than 20,000 L) can be used to produce large volumes of drug substance in many cases.
The flexibility and scalability of SUBs both facilitate process development and scale-up and have contributed to the growing use of such equipment in commercial biomanufacturing. The market-research firm also points to important drivers of that continued growth: improved return on investment (RoI) for small companies and startups, reduced complexity in automation, and positive impacts on the environment offered by SUTs (1).
In addition, a number of advances in all aspects of bioreactor technology have produced SUBs that are well suited for commercial biopharmaceutical manufacturing. These include increasingly robust films and sensors, improved bioreactor designs for power and ease of use, new stirring mechanisms with improved mixing capabilities, and enhanced automation and control systems for consistent and reliable operations.
Single-Use Bioreactors
A number of different SUB designs are used in the biopharmaceutical industry, including stirred-tank and wave bioreactors. Stirred-tank SU reactors combine the benefits of SU systems with mixing principles found in traditional stainless-steel reactors. Frame designs vary according to the type of bioreactor and a given vendor’s particular approach. For commercial bioprocessing, these systems are constructed to meet current good manufacturing practice (CGMP) guidelines. Ergonomics, ease of installation and operation, and system footprint are other factors that must be considered.
A Thermo Scientific HyPerforma Single-Use Bioreactor (S.U.B.) system includes a functional bioreactor vessel that enables mixing, venting, sparging, and temperature sensing and includes ports for monitoring, analytics, sampling, and liquid transfer. Such equipment can be configured and integrated with different commercially available control units. Integrated S.U.B. systems also come as stocked items.
Single-use BioProcess Container (BPC) bags used with S.U.B. systems are made from specialty films that are engineered for bioprocessing applications. Thermo Scientific Aegis5-14 and CX5-14 films are five–layer, 14 mil, coextruded film produced in a CGMP facility. Their outer polyester elastomer layer is coextruded with an ethyl vinyl alcohol (EVOH) barrier layer and an ultralow-density polyethylene product-contact layer. Both films are manufactured using components of nonanimal origin.
A range of available configurations fit different intended applications, and customized designs can be developed if needed. Thermo Fisher Scientific also has adopted an open-architecture approach for its BPCs, qualifying a number of SU sensor, tubing, filter, and connector suppliers to allow for greater customer input into BPC custom designs.
Scale-Up and the Turn-Down Ratio
For most cell culture applications, a 1:5 ratio is used for scaling up production processes. For example, cells first are grown in a 2-L shake flask or glass bioreactor, then in a 10-L glass bioreactor. When conditions have been optimized at small scale, a process then moves to a 50-L bioreactor before further scaling up to 250-L and 1,000-L bioreactors, with additional optimization as needed. Thus, before commercial-scale runs can be completed in a 1,000-L bioreactor, it is necessary first to conduct a process in smaller bioreactors to ensure that cell culture performance scales appropriately and that desired cell density, viability, glycosylation patterns, and expression rates can be realized reproducibly.
If equivalent bioreactor performance could be achieved at both 20% and 100% working volumes, then it would be possible to reduce the number of bioreactors required for scaling up. Historically, stirred-tank SUB designs did not facilitate 20% working volumes; their working volumes ranged between 50% and 100% of their rated volumes.
Addressing the Working-Volume Issue: To determine the cause of such reduced performance, Thermo Fisher investigated the functioning of cell cultures in S.U.B. systems with different working volumes. This required evaluation of changes in numerous operating parameters under a range of different culture conditions.
At a 20% working volume in S.U.B. systems with top overlay and bottom spargers, the top sparger does not effectively flush the large volume (80% of the bioreactor’s total volume) headspace filled with metabolic gases and carbon dioxide (CO2). Under those conditions, the gas velocity is too low above the liquid in the bag. The solubility of CO2 is higher than that of other gases, so it blankets the surface of the culture, leading to both increased CO2 dissolution and dampening of oxygen transfer. The result is a significantly negative effect on cell culture performance.
Also at a 20% working volume, the S.U.B. impeller is oversized and its shaft position is not optimized, which causes increased fluid deflection off the reactor bottom and a proportionally large mixer shear zone. So the team determined that its sparging approach needed to be modified. The probe, overlay, and sensor locations also proved to be important. A bottom water jacket is preferred for temperature control, and a drilled-hole sparger (DHS) should be used as the primary control mechanism for dissolved oxygen (DO) control.
The Solution — Crossflow Sparger Technology: Using that information, Thermo Scientific designed a new HyPerforma S.U.B. with a 5:1 turndown ratio. This new bioreactor contains a novel, patented crossflow sparger in addition to bottom drilled-hole and top overlay spargers. (Note that the overlay sparger is not used when this bioreactor operates at 20% working volume.)
Through extensive studies, the company has determined optimum operating parameters for its crossflow sparger based on O2 delivery and CO2 stripping performance. Those values were determined by measuring kLa using the “dynamic method,” an inexpensive yet repeatable and consistent analytical technique. The test solution contained 1 g/L poloxamer 188 and 3.5 g/L of pH 7.25 HEPES buffer (at air saturation).
For these cell culture studies, the investigative team used GIBCO Freedom CHO-S cells designed for MAb production. Reagents included GIBCO Dynamis Advanced Granulation Technology (AGT) medium and 0.1% GIBCO anticlumping agent. The reactor was fed with EfficientFeed C+ AGT supplement to maintain a concentration factor of 2×, a 15% D3-10 solution at constant feed, and a 45% glucose solution at constant feed (as needed to maintain a concentration of <5 g/L).
With the bioreactor operating at 20% working volume, its crossflow sparger was set to 8–10 L/m2/min. After a seed culture grew at 20% volume, the bioreactor working volume was expanded to 85%. Before that increase, the crossflow sparger was closed, and the drilled-hole and overlay spargers were opened. Agitation was maintained at 20 W/m3 for both working volumes.
Results revealed that when this bioreactor operates at 20% working volume, its crossflow sparger is effective only when positioned within 12–15 inches of the liquid surface (Figure 1). The kLa values for CO2 stripping declined when that sparger was located >12 inches above the liquid surface regardless of the gas flow rate. Even increasing the flow rate by a factor of 5× did not maintain desired kLa values.
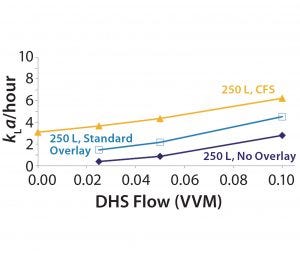
Figure 2: CO2 stripping improvements in a 250-L SUB, 5:1, 40 W/m3
In that location and with the indicated flow-through rate, the crossflow sparger introduces gas just above the liquid height for efficient oxygen delivery combined with effective sweeping of CO2 from the cell culture and flushing of metabolic gases from the overhead space. A DHS fine-tunes the DO and pH of the cell culture fluid. Thus, the bioreactor offers an added benefit of reduced bubble sparging and associated foam damage to cells.
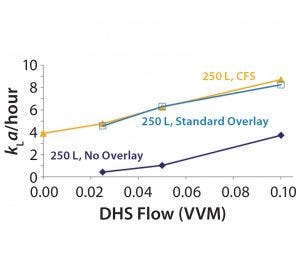
Figure 3: Oxygen transfer in a 250-L SUB, 5:1, 40 W/m3
Other design improvements in the 5:1 turndown-ratio S.U.B. system include
shaft angle adjustment (from 19.6° to 16.5°)
6-cm to 16-cm shaft length increase depending on bioreactor size (50 L to 500 L)
lower probe cutouts installed for accurate measurement of pH, DO, and temperature
a bottom water-jacketed system for improved heat transfer and temperature control at lowered volumes.
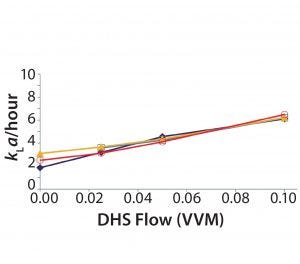
Figure 4: CO2 stripping for 50-L, 100-L, 250-L, and 500-L SUBs, 40 W/m3
When the system operates at 100% working volume, its crossflow sparger is closed and its overlay sparger is opened.
Demonstrated Results
To confirm the impact of the crossflow sparger on CO2 stripping performance, the investigational team conducted similar runs using the crossflow sparger alone, the overlay sparger alone, and no overlay sparger (Figure 2). Using the crossflow sparger, cell culture performance at 20% working volume is equivalent to that at 100% working volume. Similar results were obtained for oxygen transfer (Figure 3) from cell culture runs with 100% and 20% working volumes.
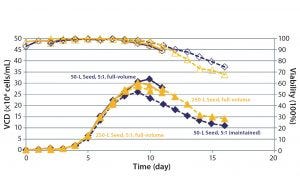
Figure 5: Consistent cell-culture performance
The crossflow sparger technology also was shown to be scalable, as illustrated by the CO2 stripping performance of 50-L to 500-L 5:1 S.U.B. systems (Figure 4). Note that viable cell density (VCD) was similar for cultures with different working volumes using appropriate sparger technology (Figures 5 and 6).
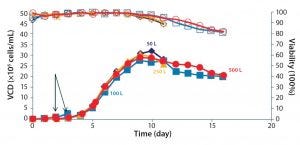
Figure 6: Scalable cell-culture performance
Benefits of a 5:1 Turn-Down Ratio
The ability to run cell cultures at both 20% and 100% working volumes in a single bioreactor facilitates both seed expansion and scale-up. Scaling a cell culture process from 2-L to 1,000-L volumes requires only a 50-L bioreactor for seed expansion. Both the 50-L and 1,000-L bioreactors can be operated at 20% and 100% working volumes, allowing for scale-up from 2 L to 10 L to 50 L to 250 L to 1,000 L using only three bioreactors (rather than five). Eliminating the need for 10-L and 250-L bioreactors reduces required capital investment and operating expenses (related to BPCs, labor, energy, and time). Also important is a reduction in the time required for process development and scale-up, which ultimately enables a faster time to market.
Additional benefits of the 5:1 turndown-ratio bioreactor include greater standardization of parts; improved use of floor space using fewer vessels in a single seed train and performing concurrent cell runs in parallel vessels; fewer solution transfers and sterile lines; and homogeneous mixing during scale-up and throughout harvesting.
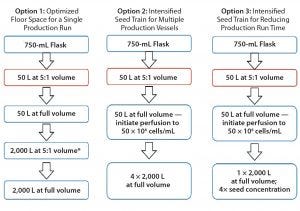
Figure 7: New options for scaling cell-culture processes
The effectiveness of crossflow sparging technology also creates an opportunity to pursue new scaling strategies for cell culture processes. For instance, if floor space is an issue, then one strategy can be pursued to optimize that footprint for a single production run. An intensified seed train for multiple production vessels also could be implemented for perfusion processes in a 50-L S.U.B. system for high-density seed cultures. Alternatively, an intensified seed train could be used to reduce overall production run times. Figure 7 presents flow diagrams for the different options.
Automation for Enhanced Performance and Ease of Use
New 5:1 turndown-ratio bioreactors containing the proprietary crossflow sparger technology for efficient cell culture come in 50-L, 100-L, 250-L, 500-L, 1,000-L, and 2,000-L sizes. These integrated SUBs are designed as complete out-of-the-box jacketed systems, each paired with an integrated controller and temperature control unit (TCU). They are fully integrated with a control system based on Emerson’s DeltaV software, considering the needs of users who require rapid setup of standard systems.
The new bioreactors have the same mixing technology and ergonomic features of other HyPerforma systems. Systems can be automated with controls for changing from the crossflow sparger to the overlay sparger when switching from 20% to 100% working volume. At 20% working volume, the crossflow sparger is opened with an appropriately set gas flow rate. At 100% working volume, the crossflow sparger is closed and the overlay sparger is opened. System automation helps to ensure that correct sparging conditions are used for each run while simplifying reactor setup.
Smart controller technology was added to Thermo Fisher’s portfolio in February 2017 through the company’s acquisition of Finesse Solutions, Inc., a developer of scalable control automation systems, sensors, and software designed to optimize bioproduction workflows. TruBio DV bioreactor control software leverages SmartParts components. It can automate a broad range of bioreactors, from 0.5-L bench-scale glass to 2,000-L CGMP SU production vessels. So the same platform can be applied from process development to commercial production. Since its launch in 2007, the TruBio DV program continues to be powered by a DeltaV controller from Emerson Process Management. It has been enhanced to leverage Finesse’s SmartParts family of intelligent digital actuators and is preconfigured with new modules for high-accuracy gas and liquid delivery into upstream bioprocesses.
SmartMFC algorithms improve flow accuracy while minimizing energy consumption. SmartPump algorithms enable gravimetric pump dosing with scale feedback or feed functions (including continuous, ramp, and bolus). Tridundant pH/DO and dual pressure sensors can be incorporated in any combination of traditional and single-use formats. In addition, third-party sensors can be integrated through analog output or the Modbus serial communications protocol. Object Linking and Embedding for Process Control (OPC) communication connects all sensors with off-line analyzers and sampling systems. TruBio offers the only truly “open” control environment for process development and scale-up without sacrificing the ability for CGMP technology transfer.
Simplifying Scale-Up
To facilitate process development and scale up, Thermo Fisher Scientific has developed a novel crossflow sparger technology that makes it possible to achieve similar cell culture performance whether running a SUB system at 20% or 100% working volume. HyPerforma 5:1 single-use bioreactors are bottomjacketed for improved heat transfer, with a bottom-drilled hole sparger that provides good mass transfer.
Experiments have demonstrated scalable mass transfer among different vessels and repeatable cell growth with no loss of performance using this new bioreactor design. The technology should benefit biopharmaceutical manufacturers during R&D, process development, and CGMP manufacturing. With an ability to perform 5:1 cell culture runs using a single vessel, it will be possible to use intensified seed trains with fewer vessels. Not only are both capital and operating expenses reduced, but development and scale-up times also can be reduced using a broad range of intensified scale-up strategies.
Reference
1 Bioproduction Market Assessment. Health Advances: Boston, MA, May 2016.
Benjamin Madsen is process development engineer 2 in advanced technology; Jeff Hurd is an engineer 3 in systems design; Chris Brau is a scientist 3 in cell biology; and corresponding author Nephi Jones is senior manager of research and development; all in single-use technologies at Thermo Fisher Scientific;[email protected].














