In an increasingly competitive life-science landscape that includes numerous mergers, acquisitions, and changing business models, the demand for collaboration is increasing at such a pace that it exceeds information technology (IT) capabilities. The need to manage and control this collaboration across the supply chain has become mandatory. That is particularly true for larger organizations with hundreds or thousands of partners that are finding new ways to connect, interact, and conduct business. Individual businesses are forming contractual affiliations that extend beyond the physical walls of their companies. Such virtual organizations are made up of many partners and suppliers. Together they make up an intricate web of activities that are necessary to conduct business and to innovate.
This transformation is driven by a number of factors, including the need to reduce costs, take advantage of geographical differences, and adapt to new requirements for the development and supply of biologics. Today’s extended enterprise includes contract research organizations (CROs), contract manufacturing organizations (CMOs), and other external entities. All work together to carve out unique business models and become more nimble than competitors (Figure 1).
Figure 1: The collaborative ecosystem
As the demand for collaboration with external partners accelerates, IT departments struggle to keep pace, so some business managers establish their own collaboration solutions. Rather than follow established methods for communication and collaboration that comply with well-established corporate policies, they often take matters into their own hands. That is why rogue business processes can spring up with questionable auditing procedures. New relationships might be based on sharing CDs and paper-based documents through conventional postal delivery services or sharing files using FTP, email, network drives, or file-sharing solutions that have been made available in recent years.
Such unregulated and unmanageable approaches expose an organization to extra risk. Intellectual property, private information, or other sensitive content can fall into the wrong hands. Often, there is little control or oversight into how information is shared or how collaborative activities are structured. Such ad hoc approaches can expose a business to potential regulatory and operational difficulties that ultimately jeopardize its reputation. These challenges are driving business leaders to consider how to conduct business practices with suppliers and partners without increasing risk or exposing their companies to breaches in regulations.
For life sciences organizations to be competitive in a challenging market, they must find efficient solutions for third-party collaborations that enable them to adapt to changes in the collaborative ecosystem. Such approaches must also allow them to focus on their core business competencies.
Cambridge, MA–based LNS Research offers this analysis: “In the highly regulated life sciences industries, the use of contract manufacturers has grown, and today’s supplier relationships have expanded in global reach and material and component complexity. As a result, the need for collaborative workspaces and effective version control has become paramount to ensure valid, safe, and legal operations” (1).
Compliance and Quality Continue to Matter
All life science organizations understand the importance of maintaining regulatory compliance and controlling quality. The industry has been bound by strict regulations for a long time. In fact, few other industries must contend with such high levels of control, transparency, and traceability. In life sciences, high levels of compliance and quality are necessary to sustain business and promote consumer confidence.
Collaboration Maturity Model
A surprising number of organizations have yet to progress beyond the use of paper and CDs to exchange final executed documents. This low level of collaboration is neither quick nor efficient, and it is hardly real time. Most life sciences companies place themselves at about level 1.5 out of 5 on the model below, and aim to be at level 3.5 within several years.
Level 1: Simple Exchange
Sharing: final executed documents
Technology: paper, email, CDs
Feels like: tactical supplier
Level 2: Secure Minimal Automation
Sharing: final executed documents
Technology: encrypted files by FTP
Feels like: sponsor to supplier
Level 3: Automated
Sharing: final documents, forms, spreadsheets, and files
Technology: shared user access (portal)
Level 4: Collaborative
Sharing: draft content, forms exchange, and systems connectivity
Technology: fully shared project sites
Feels like: great partnership
Level 5: Virtualized
Sharing: complete processes including content and data exchange
Technology: cloud-based virtual business operations
By interconnecting your systems with those of your partners, you will gain visibility into issues and be able to respond to opportunities as they arise. Nothing empowers you to maintain control of compliance and quality better than full visibility into issues from a single place in real time.
In the age of the virtual organization, how do you ensure that your partners are enforcing the same quality mandates that your organization is required to uphold? Attempting to manage it case by case, with periodic reviews and audits of partners’ operations tends to be ad hoc and inconsistent. That approach clearly will not scale as a partner network grows. Such iterative and haphazard methods also do not add up to consistent quality control.
How can business leaders ensure that employees within their purview are collaborating on the correct initiatives, supplying correct information, and managing relationships with partners in a way that reflects an organization’s overall strategy? How do sponsors ensure traceability around all of the actions that their partners and suppliers carry out on their behalf?
Countless “homegrown” solutions have sprung up in attempt to handle such new challenges. But those solutions typically require a high degree of in-house development, management, and maintenance. It is very difficult for organizations to focus on their core business while trying to build custom systems for managing collaborations. If such organizations can partner with a technology vendor that understands the business need and has a ready-made solution to meet today’s challenges, then they can take a number of giant steps forward.
Measuring Collaboration Maturity
As business evolves and a partner ecosystem expands, many life sciences organizations find it difficult to manage the transition of quality control activities to external entities or to assess the risk of such activities. It’s not easy to measure change in risk to the business that occurs when something that was previously done in-house moves outside an organization. If you have difficulty quantifying such changes, then you’re certainly going to have difficulty managing them.
Life-science companies embracing collaboration may find it helpful to apply a framework around which to structure thinking and planning. A collaboration maturity model allows business leaders to assess where their organization is in the collaboration maturity continuum and where they believe it should be in the future. The
model should describe a broad range of activities, from “light collaboration” encompassing tactical exchange of final executed documents in paper, email, or CD form to virtually integrated, cloud-based processes for securely exchanging content and ideas. (see “Collaboration” box).

Figure 2: Unregulated approaches to collaboration can expose the organization to extra risk.
It is challenging to attain high levels of collaboration maturity, especially in the fast-paced world of mergers and acquisitions. But if you don’t aim high or understand the end goal, you will get left behind. Leading life sciences organizations quickly formulate a comprehensive collaboration strategy that influences every aspect of how they do business.
For example, you should establish systematic methods for bringing suppliers on board, with end-to-end processes that dictate each company’s responsibility. That helps you manage every interaction with those partners despite differences in geography and culture. Their processes become part of your processes — and they are required to conform to your rules (
Table 1).
Are Your Systems Effective?
IT leaders should continuously assess effectiveness of their information systems. “Yes” answers to the following indicate that you are on the right track.
Do updates reach recipients in real time?
Can you confirm that updates were read and when they were read?
Can you answer auditors’ questions in real time by getting information from the system?
Are projects completed quickly?
Do you have real-time visibility into the status of corrective and preventive actions (CAPAs), deviations, and other actions?
Is the performance of your supply chain predictable?

Table 1: Collaboration maturity model
Optimizing Information Systems
US and European regulatory authorities have strict mandates on how electronic systems and records must be managed. Life-science organizations are continually reviewed to ensure that they comply with relevant regulations. But if all of your competitors have similar systems, how do you innovate? How can you leverage technology to give you an advantage?
It’s not a question of whether you have the systems. It’s a question of how efficient and well integrated those systems are. The goal is to establish a holistic environment that lets you comply with regulations, manage risk, and ensure the appropriate level of quality cost-effectively (see the “Are Your Systems Effective?” box)
The technology solutions for automating partner communication and compliance have evolved quickly. Until recently, these solutions focused on business processes that arose within the enterprise and were conducted behind a firewall. Today, life sciences companies are responsible for all of the interactions with their partners, and many interactions cross corporate boundaries. Compliance vendors must provide solutions for virtual enterprises that extend beyond a firewall yet still enforce local regulations and uphold corporate security policies.
The best solutions manage collaboration in a structured and controlled way, with complete traceability. Such information system solutions must enable sponsor organizations to be responsible for all partner activities, even though they take place outside the confines of their organizations.
Extend Existing Knowledge to Bring Partners Closer
Historically, organizations with high levels of responsibility for compliance and quality used in-house quality management systems and control management systems for ongoing quality monitoring. It is logical to extend those systems to partners and to create networks that leverage proven methods.
Because of the geographical dispersion of the life-science ecosystem, a key aspect of any modern solution is to be able to securely leverage internet technologies to achieve cost-effective systems of interaction. Ubiquitous connectivity allows partners to interact and be part of the broader compliance and quality management objective, solving the problem of expanding systems outside of the firewall.
Virtual organizations need systems that enable sponsors and partners to interact and collaborate on the same technology stack at the same time. The goal is to leverage technology and create an environment that feels as if users are in the same building, on the same floor, even though they are operating in different time zones and are part of different cultures. Only software-based solutions can provide that type of richness and the timeliness that is so critical in business.
Life sciences companies should not start from scratch when they include collaboration partners in overall conversations. They need to leverage the experiences they have with their in-house systems. Much of the understanding and efficiencies such organizations have developed are based on the successful implementation of those systems over many years. And even though those systems may change, much of the control, transparency, and efficiency have been built up through years of knowledge. Companies possess valuable understanding about how they control what they need to control, how they are successful in bringing products to market, in getting research approved, and other competencies. It is crucial to be able to extend those insights and processes to the entire supply chain using software.
By extension, those partners are brought closer to their sponsor organizations. They get pulled into the fold through shared use of joint systems and the collaboration that occurs on those systems.
Appropriate Tools, Role-based Collaboration
With any collaboration technology, it is important that people can interact and exchange information consistently. It is absolutely crucial to control the accuracy of information that is being presented, how it is consumed, how it is acted upon, and how it is represented. Typically, large organizations adhere to standards that govern how their software applications manage those exchanges.
A web-browser approach to collaboration streamlines interactions and provides a high level of consistency in all aspects of collaborations. A browser-based collaboration solution minimizes the need for local installation and is already a familiar tool to those who use IT systems as part of their day-to-day responsibilities.
Part of the process for bringing partners on board is a full training and familiarity with the environment before they can collaborate. Life sciences organizations should seek a software vendor that offers browser-based applications and training materials that are globally accessible. Such systems should enforce rigorous, role-based security and authorization procedures. In that way, you can centrally control who accesses your system and what information can be seen.
For example, if a partner is involved in contract research, then it can access the content and controls that are appropriate for its role of carrying out research. A contract manufacturing organization that is creating physical products for the sponsor organization will be able to access only capabilities and information that keep with its role in the supply chain (
Figure 3).
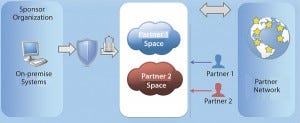
Figure 3: Applications and materials should be globally accessible and controlled by role-based security and authorization procedures.
A complete, browser-based collaboration solution can be configured to provide each type of partner with the capabilities it needs, using one version of a product. Such an approach is quite different from having to create a custom solution to reflect every individual partner’s need — a strategy that simply doesn’t scale.
Life sciences organizations with hundreds or thousands of partners need to be able to control the experiences and functions that each user accesses. It is crucial that this level of control is delivered consistently, regardless of operating system or browser type.
Partner Network Management
In the life sciences industry, the rise of the virtual organization and a focus on core competency is causing many processes to be outsourced to multiple different partners. Some sponsor organizations manage corporate networks comprising thousands of partners, suppliers, consultants, and contract organizations.
Managing compliance and quality in a partner network is an ongoing challenge. Life sciences organizations must have well-defined processes in place, supported by technology, to manage compliance and quality across extended business relationships. Companies should partner with a technology vendor that understands their business needs and has a solution to meet today’s challenges.
Author Details
Aidan Quilligan is the director of enterprise technology at QUMAS; North America: 66 York Street, Jersey City, NJ 07302; 1-973-805-8600. Europe: Cleve Business Park, Monahan Road, Cork, Ireland; 1-353-21-491-5100.
Reference
Davidson, M. 2014, 4 April. Document Management: An Enterprise Software Application Foundation. http://blog.lnsresearch.com









