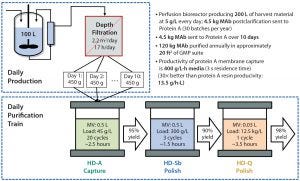
Figure 1: Right-sizing and rapid multicycling enables small-footprint downstream processing to match high-productivity upstream production (adapted from reference 1).
Filtration membranes are used extensively throughout the biopharmaceutical industry for a range of applications, from coarse filtration to nanofiltration. Advantages of filter technologies include easy scaling, disposability, and (for many membrane filters) rapid and robust performance in a single-pass. The same advantages have been realized with membrane adsorbers.
Chromatography resins are inherently disadvantaged by diffusion limits of the pores in chromatography media. Therefore, resin columns must be significantly oversized to match the performance of high productivity bioreactors. By comparison, membrane adsorbers take advantage of filter performance to deliver highly productive downstream operations. Surface-functionalized membranes from Sartorius Stedim Biotech (Sartobind Q, STIC) and Pall Life Sciences (Mustang Q)typically use anion-exchange groups for MAb polishing operations in negative mode (in which trace impurities are removed without binding the protein of interest). Such flow-through chromatography operations for impurity reduction can enhance purification productivity by over an order of magnitude: from ≤250 g/L capacity (~250 g/L-h productivity for 10 g MAb/L and two minutes residence time representing one hour operation) for chromatography resins to ≤10,000 g/L capacity (~5,000 g/L-h productivity for 10 g MAb/L and six seconds residence time representing two hours operation) for membranes.
Natrix Separations’ 3-D hydrogel membrane columns (NatriFlo HD-Q) demonstrate performance up to 20,000 g/L membrane for MAbs, with impressive host cell protein (HCP) and virus reduction with only six seconds of residence time. Membrane adsorbers are increasingly used in new flexible manufacturing facilities, in which speed of operations and robust performance are essential production criteria. These functional membranes are the first truly disposable, single-use chromatographic media with best-in-class throughput for polishing.
Membrane Chromatography: Advanced Materials
Cation-exchange operations typically show excellent aggregate reduction and HCP removal for chromatography resins. Negative-mode chromatography also can be used for significantly increased productivity. Although traditional membrane adsorbers have limited capacity, next-generation membranes have 10× more capacity than traditional membranes. Their media can be fully used in a single batch by “right-sizing” membrane columns and performing rapid cycling.
Therefore, other chromatography modalities have been developed. Sartobind S (Sartorius Stedim Biotech) and Mustang S (Pall Life Sciences) show good performance for cation-exchange chromatography, but their binding capacity is still limited by low ligand density per milliliter of membrane media. Natrix Separations is developing a salt-tolerant hydrogel membrane (Natrix HD-Sb) that can maintain high capacity and operate in either positive or negative mode (≤90 g/L or ≤ 500 g/L membrane, respectively), with good aggregate clearance.
In addition, membranes with other functional groups are in development for a range of purification operations. These include hydrophobic-interaction chromatography (Sartorius Stedim Biotech) and affinity chromatography (Sartorius Stedim Biotech and Natrix Separations). These new modalities offer full process platforms that promise to match the production rates of upstream bioreactors with appropriate downstream operations for MAb, vaccine, and virotherapy applications.
Single-Use Membrane Columns: Productivity and Flexible Manufacturing
Outputs of single-use bioreactors for commercial MAb production now range from 500 g to 20 kg at harvest. Resin columns (now offered in “single-use” prepacked format) traditionally have been used to handle such protein quantities. However, to maintain good process throughput, again the columns need to be oversized. That can be very costly especially for clinical production, in which the full resin cost is amortized over limited use. In commercial production, an expensive resin’s full useful life can be exploited.
A new paradigm in biomanufacturing involves high-productivity single-use bioreactors and a train of single-use (per batch) membrane chromatography columns that can keep pace with the upstream productivity (Figure 1). Membrane chromatography offers a powerful alternative for improved process throughput, economics, validation, and quality oversight. The strategy includes flow-through membrane adsorbers along with bind/elute membrane columns (e.g., for protein A affinity membranes) that can perform like packed-bed resins for capacity and separation but operate one to two orders of magnitude faster (2). That speed drives productivity (applied practically, not theoretically) to 30× more than resins for bind–elute chromatography.
Membrane columns can reduce operational time, cost, and complexity while maintaining high levels of production output per week for commercial biomanufacturing. A 1,000-L bioreactor culture operating at 5-g/L expression levels and a single membrane chromatography downstream train can produce 4 kg/batch of drug substance (DS). With more than one bioreactor feeding that single right-sized downstream train, production of 400 kg DS per year or more could be achieved in a fully flexible and cost-efficient facility.
Membrane chromatography is coming of age. We believe that it is the future of highly productive, flexible manufacturing for clinical and commercial protein production (3).
References
1 Jacquemart R, et al. A Single-Use Strategy to Enable Manufacturing of Affordable Biologics. Comput. Struct. Biotechnol. J. 14, 2016: 309–318; doi:10.1016/j.csbj.2016.06.0072016.
2 Hou Y, et al. Advective Hydrogel Membrane Chromatography for Monoclonal Antibody Purification in Bioprocessing. Biotechnol. Progr. 31, 2015: 974–982; doi:10.1002/btpr.2113.
3 Pollard D, et al. Standardized Economic Cost Modeling for Next-Generation MAb Production. BioProcess Int. September 2016.
Renaud Jacquemart, PhD, is principal scientist and director of vaccines process sciences; and James G. Stout, PhD, is vice president of process sciences at Natrix Separations, Inc., 5295 John Lucas Drive, Unit 6, Burlington, ON, L7L 6A8 Canada; 1-905-319-2682, fax 1-905-319-0430.








