Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

A newly opened inhouse facility gives Regenxbio not just capacity but quality and operations continuity, the firm says as it aims to bring five gene therapies to market by 2025.
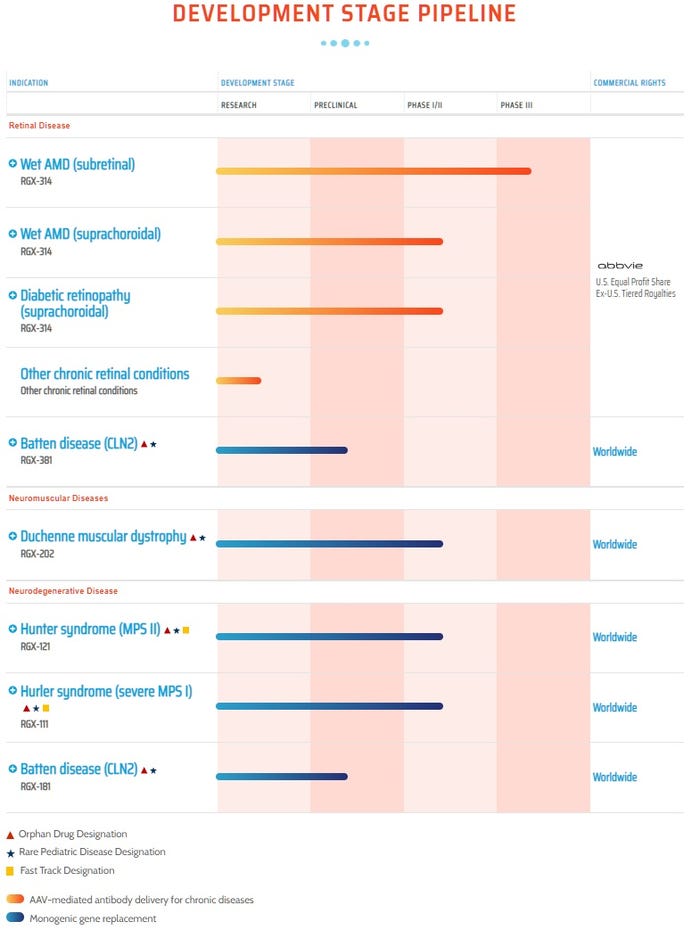
Earlier this year, gene therapy developer Regenxbio launched a strategy called ‘5×25’ aimed at progressing five adeno-associated virus (AAV) vector-based therapeutics from its internal pipeline and licensed programs into pivotal stage or commercial products by 2025.
Around the same time, the firm began operations at a 132,000 square-foot facility in Rockville, Maryland, boasting two independent bulk drug substance manufacturing suites, integrated quality control labs, and a final drug product suite.

Image: Stock Photo Secrets
Speaking during his firm’s second quarter results call last week, CEO Ken Mills described the intrinsic relationship between the two events.
“To be able to scale high-quality manufacturing, especially in a new area, you need real continuity with respect to the quality team and with respect to the floor operations team,” he told investors, adding that Regenxbio has observed “a lot of movement in people and in expertise” at contract development and manufacturing organizations (CDMO).
“That was something that, I would say, was a driver for our [inhouse] investment,” he continued, adding “that now we are more comfortable.”
Regenxbio’s reliance on CDMOs has been well documented, with the firm battling to support its pipeline of gene therapies against high demand for third-party manufacturing capacity. The firm’s CDMO network has included Fujifilm Diosynth Biotechnologies and Advanced Bioscience Labs, which it teamed up with in 2016, but began construction of the Rockville plant in 2019 to alleviate delays and take control of its manufacturing processes.
“We have an excellent quality team an excellent operations team and a team that we think is really connected to the mission of Regenxbio,” said Mills. “Having that type of opportunity at this time and place, I think, establishes that it wasn’t just about the CapEx investment, but it was about the people investment, making it something that was going to last in a sort of continuous way for us.
“We’ve got designs on taking now two pivotal programs to BLA in 2024 and we’ve got the 5×25 strategy we want to commercialize. So having that responsibility means having a stable [manufacturing network], not just a set of equipment, but a set of people as well. And I think that’s been one of the big priorities of our focus on investment.”
In Q1 2022, the firm noted a quality issue at an undisclosed third-party manufacturer had delayed clinical trial dosing for its Duchenne Muscular Dystrophy (DMD) gene therapy candidate, legitimizing the decision to invest inhouse, according to Regenxbio management.
Regenxbio’s gene therapy pipeline can be seen below:
You May Also Like