Since its inception four decades ago, cell-free synthesis (CFS) has been used to produce biomolecules such as RNA, DNA, peptides, and proteins (1). However, most of these applications have been in early stage research and small-scale proof-of-concept studies, with rare examples of large-scale production. The slow industrial uptake of CFS has been attributed to low productivity, which suggests an uneconomical path to large-scale manufacture.
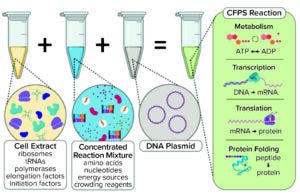
Figure 1a: Generic illustration of the key components in a cell-free protein synthesis (CFPS) reaction (4).
Typically, a CFS platform includes a genetic template (encoding the product of interest), chemical additives (nucleotides and an energy source), and a lysate (extract from ruptured cells) as prerequisites for the upstream reaction (Figure 1a). Recently, interest has grown in considering cell-free technology as a potentially attractive production platform for stratified medicine and rare disease treatments (2), on-demand medicine (3�–5), highly potent molecules (6), and difficult-to-express proteins (7). Contributory factors include significant achievements toward scalability (8), simplification of reaction mixtures (9), enhanced protocols for preparation of reaction components (10), and improvements in titer (11–13).
Ipsen Biopharm is experienced as a major manufacturer of highly potent therapeutic proteins as active pharmaceutical ingredients (APIs) for treating neurologic disorders and rare diseases (for which volumetric demands are small). Considering the above developments, CFS technology should be of benefit to Ipsen’s portfolio of low-volume, high-value biologics. That synergistic fit has led the company to harness its membership among more than 45 other participants in the industrial consortium of the Future Targeted Healthcare Manufacturing (FTHM) Hub hosted at University College London (UCL) to evaluate the applicability of CFS for biomanufacturing.
Ipsen is investigating actively the potential benefits of replacing a high-containment fermentation process with a CFS process that would provide a flexible manufacturing environment for its portfolio of highly potent neurotoxins. Here we describe insights from the user feasibility study mechanism offered by the FTHM Hub to apply decisional tools created at the UCL biochemical engineering department to help develop a business case for using CFS in production of Ipsen’s neurotoxin portfolio.
Small- and Large-Scale Applications of CFS
Commercial use of CFS technology can be classified broadly into small- and large-scale applications. Development of CFS for small-scale applications has been the main contributor to the technology’s resurgence, which comes in response to increasing demand for diagnostic reagents (14), gene/protein libraries (15), biosensors (16), high-value proteins such as neurotoxins (6, 17), and membrane proteins (18).
The nascent concept of on-demand biomanufacturing is expected to foster resilience in drug manufacturing and supply, improving access to medicines for difficult-to-reach locations and places with limited infrastructure. Some early on-demand biomanufacturing studies have shown promise. For example, Stark et al. developed a portable cell-free platform in 2019, using freeze-dried Escherichia coli lysate to synthesize efficacious bioconjugate vaccines against certain pathogens in a mouse model (5). Pardee et al. demonstrated the versatility of CFS in 2016 by using freeze-dried E. coli lysate to synthesize functional antimicrobial peptides, vaccines, and antibody conjugates (3). Continued progress in this area is projected to increase demand for cell-free technologies.
Although low productivity is currently a significant bottleneck for CFS (19), the scientific consensus is that high theoretical product yields will be more achievable with CFS than with cell-based systems (20, 21). Biosynthetic pathways can be optimized without a concomitant energy sink from secondary reactions (for example, energy expended in cell maintenance) that are inherent to cell-based systems. Process intensification methods such as continuous and semicontinuous manufacturing techniques have been adopted to resolve some productivity challenges (22, 23).
Such efforts have led to successful pilot-scale and large-scale production of biological products from cell-free platforms. In 2011, Zawada et al. showed a 106 scalability from a 250-µL to a 100-L bioreactor for production of a granulocyte-macrophage colony-stimulating factor (8). In 2012, Yin et al. developed a cell-free process that scaled from 10 µL to 5 L to produce aglycosylated antibodies and antibody fragments (24). In 2021, Ipsen researchers demonstrated a successful 10-fold scale-up of a cell-free neurotoxin production process in a 5-L stirred-tank bioreactor (6). Large-scale applications by Sutro Biopharma have shown that the cell-free platform provides potent molecules for clinical testing, having applied it to manufacture antibody–drug conjugates (ADCs) and bispecific ADC products that are currently in phase 1–1b (25).
Building a CFS Business Case
The examples above illustrate pursuits of higher product yields to help ensure a viable upstream CFS production platform. However, determining what yields are required to make an attractive business case requires a cost of goods (CoG) analysis to weigh the trade-offs in yield, process duration, and resource requirements (e.g., media and reactors). Other factors beyond yield and cost can influence decisions strongly: e.g., safety, flexibility, development timeline, and scalability. The feasibility study by Ipsen and UCL was intended to capture the differences across this broad set of financial and operational performance metrics for neurotoxin manufacturing processes relying on either cell-based or cell-free expression. The results have provided a rational basis for decision-making about an upstream production platform for a highly potent, modified recombinant neurotoxin.
We used the industrial workhorse E. coli as the template organism in a cell-based process to generate cell-free extract. UCL’s decisional tools helped us determine the CoG differences between the E. coli and CFS processes across a large matrix of possible production scenarios. We used the results to establish what level of improvements would be required for the CFS process to reach acceptable cost efficiency. To decide on the manufacturing route to adopt, we used a multiattribute decision-making approach to capture less tangible but important factors such as safety.
CoG Assessment Methodology: The process economics evaluation of CFS for highly potent modified recombinant neurotoxins was performed using a model developed at UCL’s biochemical engineering department. The model was adapted from an earlier version that initially was developed and applied to compare a mammalian cell culture with a CFS reaction for the commercial manufacture of ADCs (19). With required user-specified inputs (e.g., describing peak annual demand and dose size), we initiated a simulation in which the model performs a series of mass-balance and equipment-sizing calculations to determine necessary resources, facility footprint, capital investment, and ultimately provide a CoG estimate.
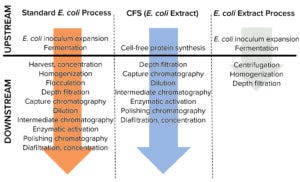
Figure 1b: Process flowsheets for the manufacture of a recombinant neurotoxin that were considered in the analysis reported herein.
Our study compares an E. coli cell-based platform process for manufacturing a neurotoxin molecule with a CFS process using either in-house or outsourced supply of cell extract. Figure 1b shows process flowsheets considered in our analysis. They indicate that processing steps are reduced with CFS, as is expression time (decreasing from 10 days to five days), compared with a cell-based process. However, that advantage does not translate to increased productivity (≈50% productivity loss) because of a fourfold reduction in neurotoxin titer. That was confirmed by Ipsen’s experimental CFS results, which showed that the maximum neurotoxin titer was 50 mg/L (6). Hence, the base-case assumption for this study supports the notion that a cell-based platform provides superior product yield, which suggests that further optimization of CFS titers would be required. When different scenarios were simulated, however, the ranking became more nuanced.
Figure 1c illustrates the scenario analysis we followed in our study, considering an annual product demand range between 100,000 and 10 million doses ranging from 10 ng to 10 μg each. We evaluated options of in-house and outsourced supply of cell-lysate extracts specific to the CFS platform. Additionally, we compared cell-based and cell-free process options across three manufacturing scales (5 L, 20 L, and 100 L) and different pipeline sizes (from a single to a multiproduct facility).
CoG Analysis: In our CoG analysis, we compared the potential of cell-free process options for the following scenarios deemed by Ipsen to be the most probable real-world cases: a 5-L upstream process, two dose sizes of 10 ng and 100 ng, and a target of 5 million drug-product vials per year (Figure 2). The general trend indicates that as dose size increases, CoG values also increase for all production platforms, so that the cost-competitiveness of CFS decreases relative to the cell-based process. However, introducing more products provides for better facility use rates and an overall reduction in CoG/dose without improving the basic competitiveness of the cell-free process relative to the cell-based process.
Figure 2(left) indicates that for single-product facilities, indirect costs (mainly facility-related overhead) represent a key CoG driver regardless of the production platform. Overhead costs associated with maintenance of process equipment and utilities, for example, remain constant regardless of use rate from 1% to 45%. For 10-ng doses, the CoG values are almost equivalent to the indirect costs, which account for >95% of the CoG across all platforms regardless of the quantity of spare product or lysate extract generated. However, for 100-ng doses, the variable costs (labor and materials) increase as facility use increases to meet the target product throughput.
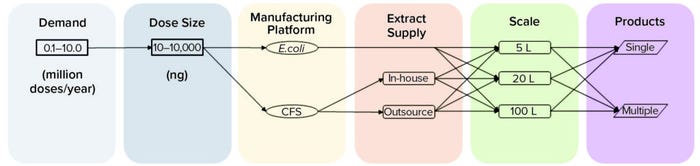
Figure 1c: Schematic representation of scenarios comparing a cell-based with a cell-free synthesis (CFS) process.
The CoG increase seen with the CFS options mainly is driven by a rise in facility use rate because their titers are lower than those from a cell-based process (Figure 1c). That suggests that manufacturing with current CFS processes is uneconomical for a plant producing one neurotoxin product at a dose size >10 ng, at which point the difference in product titer between the two options becomes a limiting factor. Based on current assumptions regarding the cost of outsourcing cell extracts and in-house performance of an E. coli extract process, both CFS options offer equivalent CoG values.
To explore CFS’s feasibility further and enhance the decision-making process for selecting an optimum manufacturing platform, we considered making multiple products (up to six) in the same manufacturing facility. The overall trend shown in Figure 2(right) indicates that CoG/dose decreases as the number of products manufactured increases for each production platform. Additionally, those results suggest that a multiproduct facility that increases facility use rates would be more economical overall because of improved allocation of indirect costs. Note that for simplicity, only the “CFSo” process with outsourced cell-extract supplies is shown in Figure 2(right).
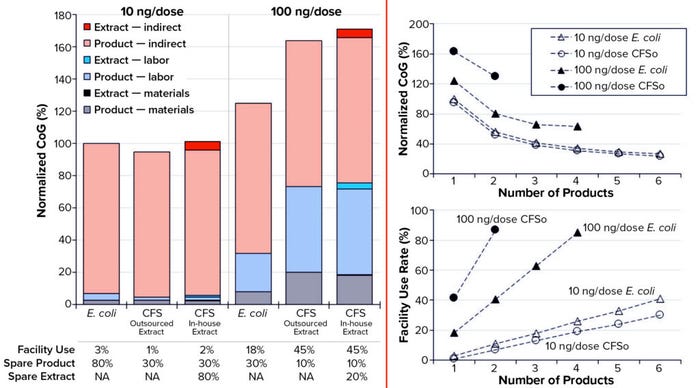
Figure 2: (left) Cost of goods (CoG) breakdown by category for Escherichia coli cell-based and cell-free synthesis (CFS) processes, the latter with an in-house and outsourced supply of cell extract, at two dose sizes (10 ng and 100 ng), and assuming an annual demand of 5 million doses from a single-product facility; (right) CoG and facility use rate for E. coli and CFS processes, the latter with an outsourced supply of cell extract (CFSo), increasing pipeline size at dose sizes of 10 ng and 100 ng.
With 10-ng doses, the CoG/dose profile is similar for both production platforms. Although the facility use rate is higher for the CFS platform, it does not exceed 45% with six products sharing the facility. Moreover, for both platforms, the reduction in CoG becomes marginal with four products or more, indicating a reverse in the CoG drivers so that variable costs dominate at that point. By contrast, the cell-based process is the better performer for 100‑ng doses, given its current status as the provider of higher product titers.
Scenario Analysis: The above analyses focused on two dose sizes (10 ng and 100 ng) and an annual product demand of 5 million doses using a 5-L bioreactor. The results in Figure 2 suggest that dose size and the number of products in the pipeline are critical parameters that influence CoG significantly. Additionally, it is expected that annual demand directly affects use rate, CoG, and potentially the manufacturing platform. Hence, we performed further scenario analysis varying annual demand, dose size, and the number of products across three production scales (5 L, 20 L, and 100 L) to elucidate the interrelationship of those factors and investigate a feasible operating window for each process platform.
Figure 3 illustrates the outcome of our scenario analysis and ranks a cell-based and a cell-free process with an outsourced extract supply. The yellow shaded part of the grid shows where the cell-based process is more cost-effective, offering at least 10% lower CoG/dose than the CFS process. The blue part of the grid shows where the CFS process is more cost-effective by at least 10%. The green part of the grid shows where both processes offer equivalent CoG/dose. Finally, the grey part of the grid shows where both processes fail to meet the target for annual product throughput. From Figure 3, it can be inferred from the diagonal split of the grids that a key parameter in ranking processes is 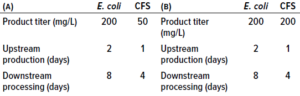 the annual product throughput (kg/year), which is derived from the product of the annual product demand (doses/year) and the dose size (ng/dose).
the annual product throughput (kg/year), which is derived from the product of the annual product demand (doses/year) and the dose size (ng/dose).
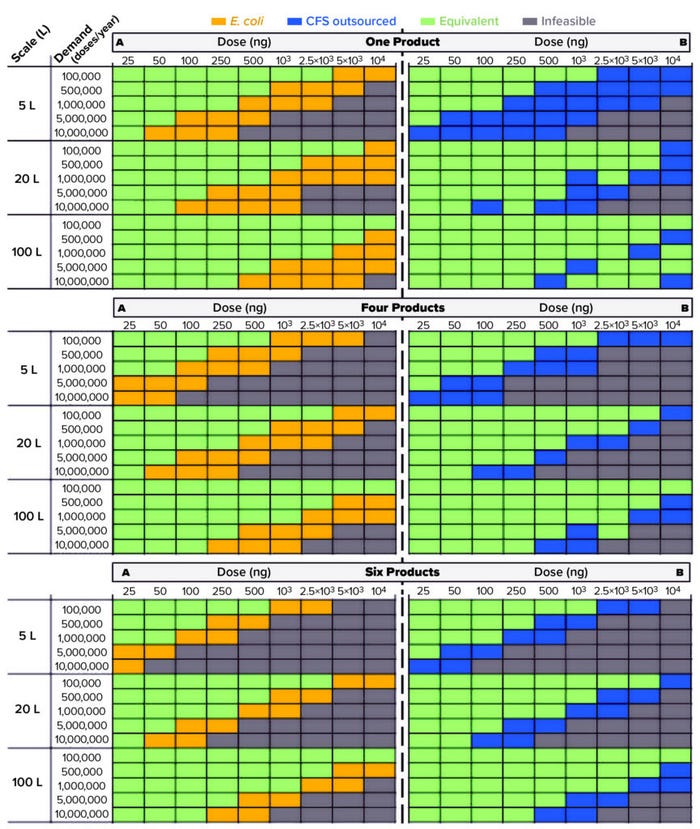
Figure 3: Scenario analysis across different combinations of the annual product demand, the dose size, the manufacturing scale, and the pipeline size for cell-based (E. coli) and cell-free (CFS) processes for (a) a base-case CFS titer of 50 mg/L and (b) an improved CFS titer of 200 mg/L; the comparison is based on CoG/dose, and the different colored areas indicate which manufacturing platform offers the lower CoG/dose. The CFS process here has an outsourced supply of cell extract. Pipeline size refers to the number of products sharing the same manufacturing facility.
Where is the operating window for CFS to outperform E. coli at current titers? Figure 3a ranks the two manufacturing platforms using our base-case assumptions (shown in the table), with the titer of the cell-based process fourfold higher than that of the CFS process. For a single-product facility, the ranking shifts as the annual product throughput increases from both processes being equivalent to the cell-based process, both being more cost-effective, and finally to both processes being infeasible. Note that although the manufacturing scale does not influence the rankings, it does have a profound impact on the infeasible area, which decreases as scale increases.
Switching from a single-product to a multiproduct facility does not change the rankings. Overall, the cell-based process outperforms the CFS process at current productivity levels across all scenarios with differing demands, dose sizes, and pipeline sizes. Hence, given current assumptions related to CFS process performance, the optimum strategy would be to keep the cell-based process and scale it up when necessary.
Where is the operating window for CFS to outperform E. coli if both systems attain equivalent titers? Figure 3b illustrates scenarios in which the CFS reaction provides a similar protein titer to that of the cell-based process, which is a potentially attainable goal for the future. That would lead to a twofold increase in productivity relative to the E. coli cell-based fermentation process. The results indicate a favorability shift towards the CFS process at that point. Moreover, increasing the CFS reaction titer to match that of the cell-based process would reduce the infeasible area and offer increased manufacturing flexibility to cover a broadened range of annual product throughputs for different neurotoxins.
So the difference in titer significantly influences which process is more cost-effective. At low dose sizes and demands, the CFS process would need a twofold improvement in titer; higher dose sizes and demands would require at least a threefold increase to offer CoG/dose equivalent to that of the cell-based process. These results will help us prioritize future process-development efforts toward the improvement of CFS titers from current levels of 50 mg/L to 200 mg/L.
Figure 4: Multiattribute decision-making analysis comparing cell-based (E. coli) and cell-free synthesis (CFSo, with outsourced cell lysate) processes, considering both operational and cost attributes; embedded table lists scores and relative importance assigned to each operational attribute considered in the analysis.
Scenario configuration: 1 million doses/year, 250 ng/dose, 5-L scale, and four neurotoxin products sharing the same facility.
Multiattribute Decision-Making Analysis: In addition to CoG/dose as a decision metric, we considered other operational factors to enhance the selection of an optimum manufacturing platform. The table embedded in Figure 4 summarizes key operational attributes considered in our analysis, with the rating achieved (1–10) for each manufacturing platform and each attribute’s relative importance. For instance, the CFS process achieved a score of 8/10 in operational ease compared with 4/10 for the E. coli process, and operational ease was given the highest importance at 100%. The rating achieved for each operational attribute was weighted based on its relative importance, and the weighted ratings subsequently were summed to calculate an operational score for each manufacturing platform. We normalized the CoG/dose values to estimate a CoG score between 0% and 100%, defining the aggregated scores as the weighted sums of the operational and CoG scores.
Figure 4 illustrates how the aggregated scores change depending on the relative importance (weight) given to operational and cost metrics. Because of its relevance to Ipsen Biopharm, the highlighted scenario is based on an annual demand of 1 million doses with a dose size of 250 ng, at a 5-L scale in a facility shared by four products. The higher its aggregated score, the more desirable is the manufacturing platform. The cell-based process was more favorable as long as the operational weight remined <65%, given the base-case titer assumption for CFS at 50 mg/L. At an operational weight of 65% (CoG weight of 35%), the CFS process (50 mg/L) breaks even with the cell-based process, and any further operational weight increases the favorability of the CFS process.
Basing a decision on CoG would suggest adopting the cell-based process if the CFS titer is limited to 50 mg/L, at which it shows slightly better scores only when the operational criteria outweigh the cost criteria. With at least a twofold titer increase (to 100 mg/L), CFS becomes the optimum strategy no matter how the financial and operational criteria are weighted. Based on this information, it becomes clear that further optimization of CFS titers will be required before any decision is made to move to a CFS platform.
The different scenarios we investigated illustrate that selecting an expression platform is a complex decision that requires evaluation of multiple factors. Disregarding the interrelation of CoG and operational elements during a production platform selection process increases the likelihood of making the wrong choice. Thus, adopting a rational decision-making approach can accelerate bioprocess development, reduce CoG, and increase the prospect of commercial success.
Feasibility Defined
Ipsen leveraged the opportunity offered by the FTHM Hub to apply UCL’s decisional tools to real-world scenarios and evaluate the potential benefits of replacing a highly contained, cell-based neurotoxin fermentation process with a CFS process to help establish a cost-effective and flexible manufacturing environment. This feasibility study focused on applying those tools to compare the process economics and the operational attractiveness of a CFS and a traditional bacterial cell-based system used for producing highly potent therapeutic proteins.
This collaboration with the FTHM Hub provided several benefits for Ipsen:
• manufacturing cost savings to improve future business performance
• process development cost and time savings
• information to evaluate shifts in investment priorities.
Specifically, the Hub’s decisional tools have helped Ipsen identify scenarios in which the cell-free platform could offer up to 35% savings in CoG. Insights from this work have helped us prioritize future R&D activities related to CFS, which is expected to reduce process development times by an equivalent of 18 person-months. Finally, this feasibility study provided critical data used to draft a business case for developing a cell-free production platform that led to Ipsen committing an additional investment equivalent to 36 person-months valued at about £0.5 million. Ultimately, the insights from this analysis have assisted critical decision-making at Ipsen by running thousands of simulations to identify optimal conditions under which the CFS platform would provide value across a matrix of scenarios, accounting for both financial and operational benefits.
Acknowledgments
We are grateful to Dr. Alistair Kippen, Alison Mason, and the senior leadership team of the bioprocess development group at Ipsen Wrexham for supporting the FTHM Hub collaboration. This work was funded by Ipsen Biopharm. We declare no competing financial interests or personal relationships that would influence the findings herein.
References
1 Pedersen A, Hellberg K, Karlsson B. Rational Improvements of Cell-Free Protein Synthesis. Nat. Biotechnol. 28(3) 2011: 218–224; https://doi.org/10.1016/j.nbt.2010.06.015.
2 Ogonah OW, Polizzi KM, Bracewell DG. Cell Free Protein Synthesis: A Viable Option for Stratified Medicines Manufacturing? Curr. Opin. Chem. Eng. 18, 2017: 77–83; https://doi.org/10.1016/j.coche.2017.10.003.
3 Pardee K, et al. Portable, On-Demand Biomolecular Manufacturing. Cell 167(1) 2016: 248–259; https://doi.org/10.1016/j.cell.2016.09.013.
4 Melinek B, et al. Toward a Roadmap for Cell-Free Synthesis in Bioprocessing. BioProcess Int. 18(9) 2020: 40–52; https://bioprocessintl.com/upstream-processing/expression-platforms/toward-a-roadmap-for-cell-free-synthesis-in-bioprocessing.
5 Stark JC, et al. On-Demand Biomanufacturing of Protective Conjugate Vaccines. Sci. Advances 7(6) 2021; https://doi.org/10.1126/sciadv.abe9444.
6 Olughu W, et al. The Systematic Approach to Developing a Cell-Free Platform Process for Recombinant Toxin Production. Toxicon 190, 2021: https://doi.org/10.1016/J.TOXICON.2020.11.454.
7 Khambhati K, et al. Exploring the Potential of Cell-Free Protein Synthesis for Extending the Abilities of Biological Systems. Front. Bioeng. Biotechnol. 7(248) 2019: https://doi.org/10.3389/fbioe.2019.00248.
8 Zawada JF, et al. Microscale to Manufacturing Scale-Up of Cell-Free Cytokine Production: A New Approach for Shortening Protein Production Development Timelines. Biotechnol. Bioeng. 108(7) 2011: 1570–1578; https://doi.org/10.1002/bit.23103.
9 Cai Q, et al. A Simplified and Robust Protocol for Immunoglobulin Expression in Escherichia coli Cell-Free Protein Synthesis Systems. Biotechnol. Prog. 31(3) 2015: 823–831; https://doi.org/10.1002/btpr.2082.
10 Yang WC, et al. Simplifying and Streamlining Escherichia coli–Based Cell-Free Protein Synthesis. Biotechnol. Prog. 28(2) 2012: 413–420; https://doi.org/10.1002/btpr.1509.
11 Caschera F, Noireaux V. Synthesis of 2.3 mg/mL of Protein with an All Escherichia coli Cell-Free Transcription-Translation System. Biochimie 99, April 2014: 162–168; https://doi.org/10.1016/j.biochi.2013.11.025.
12 Des Soye BJ, et al. A Highly Productive, One-Pot Cell-Free Protein Synthesis Platform Based on Genomically Recoded Escherichia coli. Cell Chem. Biol. 26(12) 2019: 1743–1754; https://doi.org/10.1016/j.chembiol.2019.10.008.
13 Colant N, et al. A Rational Approach to Improving Titer in Escherichia coli–Based Cell-Free Protein Synthesis Reactions. Biotechnol. Prog. 37(1) 2020: e3062; https://doi.org/10.1002/btpr.3062.
14 Pardee K, et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 165, 2016: 1255–1266; https://doi.org/10.1016/j.cell.2016.04.059.
15 Sawasaki T, et al. A Bilayer Cell-Free Protein Synthesis System for High-Throughput Screening of Gene Products. Fed. Eur. Biochem. Soc. Lett. 514, 2002: 102–105; https://doi.org/10.1016/s0014-5793(02)02329-3.
16 Soltani M, et al. Reengineering Cell-Free Protein Synthesis As a Biosensor: Biosensing with Transcription, Translation and Protein Folding. Biochem. Eng. J. 138, 2018: 165–171; https://doi.org/10.1016/j.bej.2018.06.014.
17 Zichel R, et al. Efficacy of a Potential Trivalent Vaccine Based on Hc Fragments of Botulinum Toxins A, B, and E Produced in a Cell-Free Expression System. Clin. Vac. Immunol. 17(5) 2010: 784–792; https://doi.org/10.1128/cvi.00496-09.
18 Park KH, et al. In the Cauldron of Cell-Free Synthesis of Membrane Proteins: Playing with New Surfactants. New Biotechnol. 28(3) 2011: 255–261; https://doi.org/10.1016/j.nbt.2010.08.008.
19 Stamatis C, Farid SS. Process Economics Evaluation for Cell-Free Synthesis for the Commercial Manufacture of Antibody Drug Conjugates. Biotechnol. J. 16(4) 2020: e2000238; https://doi.org/10.1002/biot.202000238.
20 Wilding KM, et al. The Emerging Impact of Cell-Free Chemical Biosynthesis. Curr. Opin. Biotechnol. 53, 2018: 115–121; https://doi.org/10.1016/j.copbio.2017.12.019.
21 Hunt JP, et al. Engineering Cell-Free Protein Synthesis for High-Yield Production and Human Serum Activity Assessment of Asparaginase: Towards On-Demand Treatment of Acute Lymphoblastic Leukemia. Biotechnol. J. 15(4) 2020: e1900294; https://doi.org/10.1002/biot.201900294.
22 Carlson E, et al. Cell-Free Protein Synthesis: Applications Come of Age. Biotechnol. Adv. 30(5) 2012: 1185–1194; https://doi.org/10.1016/j.biotechadv.2011.09.016.
23 Karim A, Jewett M. A Cell-Free Framework for Rapid Biosynthesis Pathway Prototyping and Enzyme Discovery. Metabolic Eng. 36, July 2016: 116–126; https://doi.org/10.1016/j.ymben.2016.03.002.
24 Yin G, et al. Aglycosylated Antibodies and Antibody Fragments Produced in a Scalable In Vitro Transcription-Translation System. mAbs 4(2) 2012: 217–225; https://doi.org/10.4161/mabs.4.2.19202.
25 Our Pipeline: Best-In-Class Cancer Therapuetics. Sutro Biopharma: South San Francisco, CA, 2021; https://www.sutrobio.com/pipeline.
Corresponding author Williams Olughu ([email protected]) is principal scientist, and David Gruber is director of bioprocess development at Ipsen Biopharm Ltd., Unit 9 Ash Road North, Wrexham, LL13 9UF, UK; 44-1978-661181; https://www.ipsen.com. Christos Stamatis ([email protected]) is a research fellow in the department of biochemical engineering, and Suzanne S. Farid ([email protected]) is a professor of bioprocess systems engineering at University College London, London WC1E 6BT, UK.














