Covance is one of the world’s largest and most comprehensive drug development services companies with more than 10,000 employees in 60 countries. Through its broad portfolio of services, Covance has helped pharmaceutical and biotech companies develop one-third of all prescription medicines in the market today.
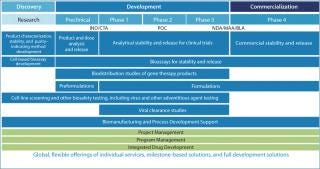
Working with clients of all sizes, Covance recognizes that your development program is as unique as the product you bring to our facilities. With over 250 different biomolecules successfully supported annually, Covance Biopharmaceutical Services provide you with a global, multidisciplinary team of experts and specialized state-of-the-art infrastructure to help achieve your development and commercialization goals.
The comprehensive Covance biopharmaceutical service capabilities include extensive expertise and experience in:
Adventitious agent testing
Covance has a complementary portfolio of discovery, nonclinical safety assessment, clinical development, and market access capabilities for full development of biopharmaceutical products.
We invite you to visit our facilities in North America and in Europe to meet our scientists and to experience first-hand the difference our proven biologics expertise can bring to your development program.
About the Author
Author Details
Ji Wu, PhD, is marketing manager for Covance, 3301 Kinsman Boulevard, Madison, WI 53704, USA; [email protected], 1-888-268-2623.