Selecting a CMOis critical to biotech biologic drug development. A key factor for selecting a CMOis experience. How do you measure experience? It can be measured in many ways, metrics include
Number of GMP processes developed by the CMO
Number of projects involving your strain
Number of projects involving your product type
GMP production success rate
Number of GMP lots produced.
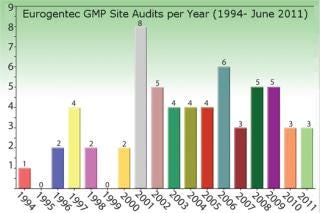
Eurogentec’s Biologics Division is specialized in the manufacturing of biopharmaceuticals from microbial sources such as bacteria, yeast, and biosafety level 2 organisms. Eurogentec manufactures in accordance with GMP since 1994.
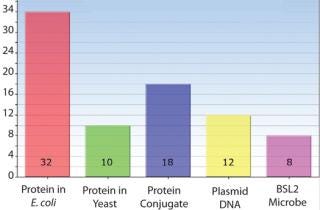
Host Cell Experience — Microbial Experts
Numerous BSL-2 organisms.
Chemistry Experience — Conjugation Experts
Other chemistries available.
Product Family Experience
Protein–protein conjugations
Protein–peptide conjugations.
GMP Infrastructure
Three GMP fermentation suites (80 L, 150 L, 500 L)
Two GMP purification suites
One GMP sterile filtration suite.
Figure 1: ()
Figure 2: ()
Figure 3: ()
Process Development Strategies
FastTrack — quick to clinic
OptiTrack — robustness built in.
About the Author
Author Details
Dr. Pascal Bolon is biologics sales and marketing manager at Eurogentec SA, Rue du Bois Saint-Jean 5, 4102 Seraing, Belgium; 32-4-366-6116; [email protected].