As a CMO partner, Avid Bioservices provides fully integrated services across the process chain to develop and commercialize a biologic including cell line and process development, analytical methods development, clinical and commercial cGMP manufacturing, and regulatory submissions. We provide first-hand expertise and knowledge to navigate a biologic from concept to commercialization because we are a wholly owned subsidiary of Peregrine Pharmaceuticals, a clinical-stage, publicly traded biotech company with a management team experienced in the development and commercialization of therapeutic proteins. This allows us to provide our clients with unique insights to manage the process, scale-up, and validation challenges of drug development and commercialization while mitigating risk, reducing costs, and accelerating time to market.
Our clients benefit not only from our comprehensive service offerings, but also from the experience that comes from navigating our own products through development. Avid has produced >160 CGMP runs in over 15 different indications:Table 1: Project history
Table 1: Project history ()
Dedicated to Production of Mammalian Cell Culture Biologics
Our fully integrated service offerings allow us to streamline the process by executing multiple parts simultaneously and effectively using the results in subsequent and ongoing processes. Through all stages we ensure seamless transitioning and provide clients with a single point of contact to simplify the dissemination of project information. We pride ourselves on being able to apply our knowledge and experience to benefit our clients and their projects throughout the process. Our manufacturing expertise spans from clinical to commercial production, including commercial API production since 2005. Development, Analytical, and Manufacturing Expertise Accelerates Time to Market
Our development teams can assist with any challenges you may be facing: from scale-up to process optimization to analyzing and characterizing your antibody or protein. A broad understanding of bioprocesses and bioreactor cell culture enables significant improvements in recovery rates, stability,and actual protein expression through upstream and downstream process development and optimization, all to accelerate the time to market. Protein characterization assures safe, stable and efficacious products, providing clients with a well-characterized biopharmaceutical product
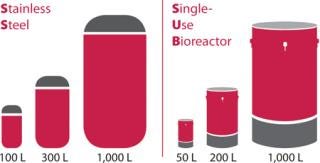
Avid’s facility currently has 2,650 L total capacity (Figure 1), with potential expansion of 6,000 l additional using single-use bioreactors (SUBs). We provide flexible manufacturing-scale solutions to meet any phase of production. Our established partnerships further advance our expertise and scale-up capacity.
Figure 1: ()
Committed to Superior Quality and Customer Service
Avid’s proven track record of quality and customer service since our inception in 2002 speaks for itself. Our successful inspection track record continues to grow, from audits by the US Fda, European agencies, and the State of California, as well as clients and other regulatory agencies. This is further supported by the robust quality systems we have in place and our dedication to produce high-quality products each and every time.
At Avid, we are dedicated to setting the standard in customer service and exceeding client expectations. Our project management team has extensive experience collaborating with clients and contributing to their success. We offer a flexible approach to project execution by tailoring each project plan to the client’s specific requirements. Throughout the process, project management acts on behalf of the client and drives the project internally and externally, ensuring that timelines are met and product is delivered on time.
About the Author
Author Details
Kelly Pisarev Lord is manager of marketing communication for Avid Bioservices, Inc., 14282 Franklin Avenue, Tustin, CA 92780; 1-714-508-6100; [email protected], www.avidbio.com.