SARS-CoV-2 Variants: A Case for Expanding mRNA Vaccine Production Globally
February 9, 2023
Vaccines based on messenger ribonucleic acid (mRNA) created headlines in December 2020 for being the first highly efficacious SARS-CoV-2 prophylactics to receive emergency use authorization (EUA) from the US Food and Drug Administration (FDA). Within a couple months of the virus’s gene sequence being published (1), Pfizer and BioNTech were ready with their vaccine candidate (2), and in less than a year, the vaccine was approved for administration in adults. EUA for Moderna’s mRNA vaccine followed soon after. The rapidity with which those products were developed stands in stark contrast to the 10–15 years that traditionally have been required for a candidate vaccine to move from discovery to approval and administration in patients.
Pfizer–BioNTech’s and Moderna’s respective vaccines were developed in such short timeframes because mRNA technology was not designed overnight to mitigate COVID-19. Scientists have been studying mRNA vaccines for almost three decades to protect against influenza, rabies, Zika virus, and cytomegalovirus (CMV) (3). The modality did not gather momentum during early studies because of immunogenicity, stability, and delivery issues. However, unprecedented global collaboration, funding, and government support relating to the COVID-19 pandemic accelerated efforts to address the difficulties surrounding mRNA technology. The outcome has been rapid availability of COVID-19 mRNA vaccines in major markets, based on work from manufacturing resources that are spread out around the world.
How mRNA Vaccines Are Made
During mRNA vaccine development, scientists first must identify a target protein from the pathogen of interest — e.g., in the case of SARS-CoV-2, the spike (S) protein. However, the target protein must be sufficiently different from proteins in human cells so that resulting immune responses detect and address only the pathogen. Hence, in the case of COVID-19 vaccines, many drug companies selected the SARS-CoV-2 S protein. Inducing antibody binding with the S protein would prevent attachment of a virus particle to human cells exhibiting the angiotensin-converting enzyme 2 (ACE-2) receptor, thereby preventing viral entry.
After target identification, scientists insert the DNA sequence coding for the gene of interest (GoI) (for SARS-CoV-2 vaccines, that is the gene encoding for the S protein) into a plasmid, which is amplified in host bacteria (typically Escherichia coli) using a fermentor (Figure 1). The resulting plasmid DNA (pDNA) undergoes purification and linearization. Plasmids easily replicate and magnify target gene sequences, enabling rapid production.
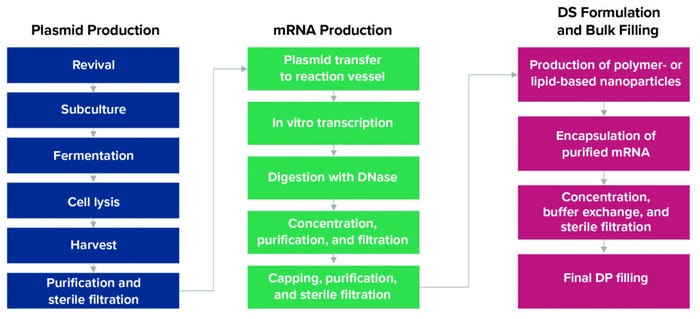
Figure 1: Manufacturing process for mRNA-based vaccines (DS = drug substance, DP = drug product).
During a cell-free in vitro transcription (IVT) process, a pDNA template is incubated with enzymes and nucleotides to produce mRNA. Additional steps are performed using distinctive enzymes to cap and thus stabilize that material. After enough mRNA has been produced in an IVT reaction, the pDNA is broken down to ensure that only mRNA will be incorporated into the vaccine.
Because mRNA is highly susceptible to degradation, a lipid nanoparticle (LNP) or other suitable delivery system must be developed to package the mRNA and deliver it to cells. After purified and sterile-filtered mRNA is encapsulated, final formulation activities occur. The resulting drug product is the vaccine. Upon administration, encapsulated mRNA is taken up by a recipient’s cells, instructing them to produce the SARS-CoV-2 S protein and thereby inducing an immune response in the form of antibody production.
Advantages of mRNA Vaccines
A Strong Safety Profile: Because mRNA vaccines do not contain live–attenuated or inactivated viruses, such products raise little risk for protein- or virus-derived contamination, infections, and unintended immunogenic reactions. After injection into a patient, mRNA degrades quickly by normal cellular processes, thereby giving it a strong safety profile with far less chance of side effects than is associated with conventional vaccine approaches. The probability of mRNA integrating into a host genome also is low, minimizing potential for inducing immune rejection reactions.
Short Manufacturing Timelines: Compared with other biological drugs, mRNA vaccines can be produced more rapidly using cell-free processes. Production can be standardized and scaled up readily.
High Efficacy Levels: Messenger RNA induces expression of specific antigens that give rise to both antibodies and T cells, resulting in effective immune responses with ~95% efficacy.
Manufacturing Flexibility: Messenger RNA can be used to encode and express many kinds of proteins. Modifying an mRNA sequence also is easier than other approaches to vaccine modification. Such flexibility provides for rapid vaccine development. Thus, despite the presence of other types of COVID-19 vaccines in the market, the flexibility of mRNA gives it a significant advantage in vaccine manufacturing today. Because of the relative ease with which target protein sequences can be changed, mRNA technology can play an important role in managing SARS-CoV-2 variants.
Regardless of the protein sequence being encoded, neither production nor purification processes require redevelopment for modification of an mRNA-based product. Thus, mRNA vaccines coding for different proteins can be produced with minimal additional process development. When variants emerge, as long as the target protein sequence is designed, making a given mRNA vaccine is relatively straightforward.
With new COVID-19 variants surfacing around the globe, the mRNA vaccine could be a large part of the solution people are expecting to end the pandemic.
Complications with mRNA Manufacturing
Ensuring global access to mRNA vaccines is easier said than done. Currently, Pfizer–BioNTech, Moderna, and their partners are the only companies in the world to have implemented mRNA manufacturing processes and commercialized the resulting vaccines. That is true, in part, because the Comirnaty and Spikevax products are currently the only such vaccines to have received FDA approval; mRNA vaccines being developed by other companies are still in clinical development.
Managing a pandemic calls for extensive vaccine availability at high speeds (to help ensure that a meaningful proportion of the world’s population can be inoculated in the shortest time possible) and with considerable flexibility (to revise the antigen being produced in response to emerging variants). Even with Moderna and Pfizer–BioNTech manufacturing their mRNA vaccines in large quantities, the volumes produced have been insufficient for vaccinating the global population quickly enough. It also is unreasonable to expect those and other vaccine manufacturers to drop other programs and jeopardize the management of other diseases.
In short, the world is learning that a handful of manufacturers cannot make new mRNA vaccines quickly enough to arrest the spread of pandemic variants such as those stemming from SARS-CoV-2. Assuming considerable reliance on mRNA manufacturing, as has been the case with COVID-19, pandemic management also will require increased understanding of mRNA manufacturing, which remains complex and riddled with obstacles.
Sensitivity to Degradation: Messenger RNA is a large, unstable molecule that is sensitive to shear stress and enzymatic degradation. Ribonuclease (RNase) degrades mRNA and is ubiquitous in the human body. Hence, it is important to minimize or even eliminate RNase activity during mRNA production to prevent degradation.
Process Complexity and Cost: IVT and purification steps are complex and expensive because of costs for requisite raw materials. Process conditions must be screened carefully to optimize IVT and subsequent separation of contaminants such as double-stranded RNA, truncated mRNA, and residual DNA. Subsequent encapsulation of mRNA into LNPs is yet another critical step to account for.
Equipment Needs: Currently, the biopharmaceutical industry lacks equipment designed to process small volumes of molecules that are significantly larger than traditional recombinant proteins. A small quantity of linearized pDNA is sufficient to produce large volumes of mRNA.
Storage and Transport Conditions: Because mRNA nanoparticles are extremely sensitive, ultracold-chain logistics are required to deliver COVID-19 vaccines to distribution sites. Currently, the Pfizer–BioNTech Comirnaty vaccine requires storage between –90 °C and –60 °C, and Moderna’s Spikevax product must be stored between –50 °C and –15 °C.
Global Vaccine Production Centers
Given the many advantages of mRNA as a vaccine modality, the world urgently needs to expand capabilities for its production. Public health experts have argued that manufacturing challenges cited by innovator companies are not insurmountable.Pandemics necessitate consideration of novel biomanufacturing strategies, including development of additional manufacturing centers in different regions to make mRNA vaccines widely available quickly and with minimal logistic challenges.
Although multiple, parallel vaccine-development efforts would be useful, sharing intellectual property (IP) to manufacture authorized/approved vaccines would speed up vaccine availability substantially and facilitate pandemic management. Vaccinating early and broadly can arrest development of variants. Sharing mRNA-related IP as is common between clients and service providers would provide a viable solution. Such a system would benefit millions of people who otherwise would be left unvaccinated and exposed to viruses and their variants.
However, today, manufacturing technology for COVID-19 mRNA vaccines remains the exclusive preserve of a handful of companies and their supply-chain partners, leading to biased vaccine distribution. That result runs up against the fact that COVID-19 vaccines were supported by vast amounts of public funding, collaboration, and knowledge-sharing meant to benefit society at large.
Given what circumstances have materialized during the pandemic, we must establish a global legal framework that incentivizes and/or mandates companies to share technologies and manufacturing know-how that are developed as part of a shared pandemic response. Such a framework would enable global production and, consequently, equality in vaccine access.
Contract research and contract development and manufacturing organizations (CROs, CDMOs) that have deep scientific expertise in biologics manufacturing can be ideal partners in the drive to expand global vaccine production. For example, Moderna’s Spikevax product has reached millions of people in the United States because the company partnered with CDMO Catalent to manufacture that vaccine.
Most biologics CROs/CDMOs already have much of the equipment and capacity, workforce, and technology-transfer experience required to execute such programs successfully. For instance, my company, Syngene, has expertise in biologics discovery, cell-line development, process development, and manufacturing for clinical and commercial supply of a diverse set of large molecules. By hiring competent CROs/CDMOs from around the globe, vaccine developers can expand production quickly, benefiting millions of people who are deprived of emerging treatment modalities that are critical for pandemic mitigation. According to the United Nations Children’s Fund (UNICEF), only 11% of people in low-income countries had been vaccinated as of April 2022 (4). In the World Health Organization’s (WHO’s) Africa region, 83% of people remained unvaccinated, and 51% of people in the Eastern Mediterranean region, including Afghanistan, had yet to be inoculated.
India’s Capability for mRNA Vaccine Production
India is known as “the world’s pharmacy” because of its ability to manufacture innovative, lifesaving medicines at affordable prices for countries in particular need. Thus, it is a logical place to start establishing regional centers for mRNA production. The Indian government recently announced development of its own COVID-19 mRNA vaccine, produced jointly by its Centre for Cellular and Molecular Biology (CCMB) and Council of Scientific and Industrial Research (CSIR). Early in the pandemic, India was among the first countries to develop an inactivated SARS-CoV-2 vaccine (Covaxin). India’s prowess in vaccines and biologics is demonstrated further by the successful launch of the Covaxin product by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) National Institute of Virology (NIV).
Biopharmaceutical manufacturing also requires government support, substantial capacity, and stringent systems for regulatory compliance. India stands tall in all three respects. It accounts for 60% of vaccine supplies delivered to UNICEF (5). India also manufactures four of the WHO’s approved COVID-19 vaccines. That work amounts to five billion vaccine doses administered in 2022. So far, India has exported 200 million doses of COVID vaccines to 100 countries (5). By being at the forefront of COVID-19 vaccine research and development (R&D), India has cemented its position as one of the largest vaccine manufacturing hubs in the world, with a ready talent pool and a robust life-sciences ecosystem.
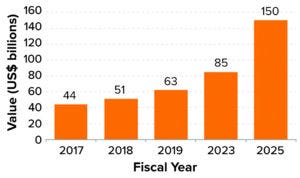
Figure 2: Market value of the Indian biotechnology industry (6).
Also of note is that the Indian biotechnology industry, which was valued at US$63 billion in 2019, is expected to reach $150 billion by 2025, with a compound annual growth rate (CAGR) of 16.4% (Figure 2) (6). Similarly, the Indian biologics market is forecasted to reach $12 billion by 2025, at a CAGR of 22%. The industry comprises around 5,000 biotech companies, including 4,240 start-ups and 760 core biotechnology companies.
An Urgent Global Need
COVID-19 vaccines based on mRNA have proven to be safe and highly efficacious, with strong scalability and brief development periods. Messenger RNA platforms also exhibit considerable flexibility, enabling rapid launch of new vaccines against emerging pandemic variants. With nearly 6.6 million lives lost to COVID-19 (as of 20 November 2022), there is an urgent need to expand vaccine production capabilities globally (7). Such an approach would go a long way in safeguarding the world from dangerous mutations while making vaccines cost-effectively and helping to ensure their availability worldwide.
India has a sizable biologics production capability and a strong track record in vaccine production. As countries in the northern hemisphere seek to manage a resurgence of the virus in the winter months, governments are learning that they must work together to build global vaccine R&D and production networks to protect against future public health crises. To quote epidemiologist Larry Brilliant, “Outbreaks are inevitable, but pandemics are optional” (8). We have no time to lose in preparing for the outbreaks ahead.
References
1 Sequencing of SARS-CoV-2: First Update. European Centre for Disease Prevention and Control: Stockholm, Sweden, 18 January 2021; https://www.ecdc.europa.eu/sites/default/files/documents/Sequencing-of-SARS-CoV-2-first-update.pdf.
2 Agrawal G, et al. On Pins and Needles: Tracking COVID-19 Vaccines and Therapeutics. McKinsey & Company: Insights, February 2021; https://www.mckinsey.com/industries/life-sciences/our-insights/on-pins-and-needles-will-covid-19-vaccines-save-the-world.
3 Sahin U, Karikó K, Türeci Ö. mRNA-Based Therapeutics: Developing a New Class of Drugs. Nat. Rev. Drug Discov. 13(10) 2014: 759–780; https://doi.org/10.1038/nrd4278.
4 We Must Take the Rapid Action Needed To Accelerate Vaccination. UNICEF: New York, NY, 12 April 2022; https://www.unicef.org/press-releases/we-must-take-rapid-action-needed-accelerate-vaccination.
5 COVID-19 Vaccine Supply. India Ministry of External Affairs: New Delhi, India, 16 November 2022; https://www.mea.gov.in/vaccine-supply.htm.
6 Biotechnology Industry in India. India Brand Equity Foundation: New Delhi, India, August 2022; https://www.ibef.org/industry/biotechnology-india.
7 Coronavirus (COVID-19) Dashboard. World Health Organization: Geneva, Switzerland, 20 November 2022; https://covid19.who.int.
8 Brilliant L. Sometimes Brilliant, in Conversation with Stewart Brand. The Interval: San Francisco, CA, 21 February 2017; https://theinterval.org/salon-talks/02017/feb/21/sometimes-brilliant-conversation-stewart-brand.
Mahesh Bhalgat is chief operating officer of Syngene International Limited, Biocon Park, SEZ, Bommasandra Industrial Area — Phase-IV, Jigani Link Road, Bangalore 560 099, India; https://www.syngeneintl.com/contact-us.
You May Also Like




