- Sponsored Content
- Manufacturing
Tools for Simplifying MSC Expansion, Cryopreservation, and DMSO Removal
June 1, 2021
Date: Jun 1, 2021
Duration: 20 Min
Sponsored by Corning
This webcast features: Hilary Sherman, Senior Scientist, Corning Life Sciences
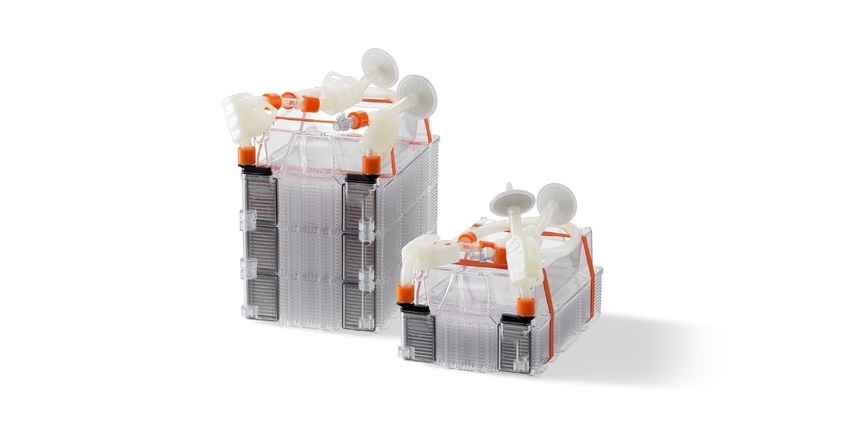
Mesenchymal stem cells (MSCs) are one of the most frequently utilized cell types for cell therapy applications due to their ability to be isolated from several sources and easy adaptability to culture conditions. Bone marrow derived MSCs in particular are commonly studied due to their ease of access and achievable therapeutic dosage. While expanding MSCs to achieve these quantities, there is a risk for heterogeneity-induced quality failures during the manufacturing process. Utilizing a manufacturing process that maintains a homogeneous MSC population to meet required critical quality attributes can lead to greater clinical success. Corning’s HYPERStack platform allows for large-scale expansion in a minimal footprint while generating high-quality, homogeneous MSCs.
This webinar will focus on the following key points that are critical to understanding how to achieve large-scale MSC expansion:
Choosing the correct platform for manufacturing homogeneous MSCs
Considering closed system process design during manufacturing
Showing consistent bone marrow derived stem cell surface marker expression in application data during cell culture scale-up
Just fill out the form below to watch the recorded webcast.
About the Author(s)
You May Also Like





