Presented by: Willie Hesselink, technical application project manager,
biopharmaceutical production, Europe, Avantor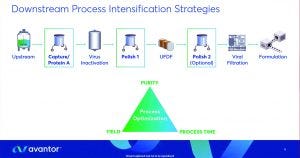
Investments in cell-culture technologies have increased monoclonal antibody (MAb) yields significantly over the past two decades. Downstream operations continue to lag in productivity, however. Hesselink explored how companies can align downstream productivity with upstream yields to mitigate production bottlenecks. He focused on intensifying chromatography steps, optimizing buffer preparation, and mitigating variations across raw materials.
Chromatography resin selection is vital to downstream intensification, Hesselink explained. Capture and polishing steps influence MAb recovery considerably and incur higher costs than other downstream activities. Selecting an appropriate resin can maximize yields and improve target purity. He demonstrated the utility of resin-optimization studies by describing Avantor’s work with a company developing an IgG1 MAb expressed using Chinese hamster ovary (CHO) cells. The drug sponsor had struggled to achieve target purity levels, instigating production bottlenecks. Avantor determined that the sponsor’s process could be improved using PROchievA protein A affinity resin for antibody capture, a Bakerbond PolyCSX strong cation exchanger for intermediate polishing, and a Bakerbond PolyQUAT strong anion exchanger for final polishing. The optimized protein A step achieved MAb purity levels of ~95%. After intermediate and final polishing, MAbs showed 99.9% purity, with yields >90% and no significant product loss.
Downstream processing can be optimized further by streamlining buffer preparation. For instance, vendor-enabled dispensing systems for bulk dry powders can reduce or eliminate many sampling and testing requirements associated with conventional preparation methods. Avantor’s modular and custom-weight J.T. Baker systems enable controlled dispensing of ready-to-use powders, and the systems are compatible with Raman identification technologies. Traditional preparation, Hesselink explained, can require 30 hours from receipt of materials to hydration. By leveraging preselected bag sizes and specified dry-powder volumes, preparation using a modular J. T. Baker system can take 12 hours. Avantor’s custom-weight option for large-scale operarions requires only nine hours because its components arrive preweighed.
Liquid-formulation buffers can streamline preparation further still. The simplest option is to outsource standard-concentration (1×) buffers that can be delivered to storage tanks or chromatography skids. Alternatively, companies can purchase buffer stocks that can be blended with acids and bases. Although that approach is costlier than outsourcing 1× formulations, it increases user control over buffer pH and conductivity.
A promising option is to hydrate buffer concentrates using an in-line dilution (ILD) skid. Avantor has collaborated with Ireland’s National Institute for Bioprocessing Research and Training (NIBRT) to develop such a system. Avantor’s model features an in-line static mixer and surge-vessel mixing as well as accurate weight and flow meters. Because the skid uses disposable components, it requires no cleaning validation. Like a buffer-blending system, Avantor’s ILD unit increases user control over buffer parameters. It also can decrease facility requirements and capital expenditures significantly.
Hesselink concluded by providing strategies for mitigating raw-material variability. Although real-time evaluation is unavailable, emerging technologies for process monitoring and data analytics enable tracking of raw-material quality over time. Avantor is developing solutions that aggregate and analyze data from stability interval testing of buffers, resins, excipients, and other downstream components. Such data could empower companies to anticipate variations, increase supply-chain visibility, augment understanding of a variation’s process impacts, and minimize batch failures.
A primary challenge is to analyze such data in ways that inform process and supply-chain decisions. To that end, Hesselink mapped out different raw materials, their potential for variability, and the impacts of such deviations. Impact is ranked as low, moderate, or high depending on a component’s proximity to a patient, the criticality of the associated process step, and the level of supplier engagement that is needed to monitor its quality. For instance, excipients pose moderate variability risks, and they have high impact because they come into direct contact with patients during administration. Thus, it makes sense to evaluate excipient-stability data closely and engage suppliers to mitigate variability concerns.






