Cell and gene therapy manufacturers need to ensure the safe delivery of their products and rely on fast and reliable immunoassay platforms to do so.
This white paper gives an overview of the viral vector landscape and the requirements for immunoassays in viral vector bioprocessing analytical workflow. It also shares case studies from viral vector labs that are using the Gyrolab technology to deliver fast and reliable results.
Increasing productivity in cell and gene therapy bioprocessing with fast and reliable immunoassays
The timely release of safe and efficacious cell and gene therapeutics depends on many factors. One is the availability of analytical platforms that can quickly deliver reliable data on viral titer and process-related impurities.
There are many factors to consider when choosing an platform to ensure high productivity. The system must be robust and quickly generate reliable data from small amounts of precious sample. It should enable rapid method development and still provide flexibility to support the development of novel assays.
Automation and a walk-away systems are essential to reducing hands-on-time and minimizing manual errors, enabling personnel to spend time on more important tasks. Added to that, ready validation and a software that enables 21 CFR Part 11 compliance is a must to meet regulatory guidelines.
We will investigate the needs of immunoassays in bioprocess analytics for cell and gene therapy in detail and how increased productivity can be met.
Immunoassays such as ELISA are frequently used to measure critical quality attributes, such as virus titer and impurity levels, but can be laborious and consume large amounts of precious sample.
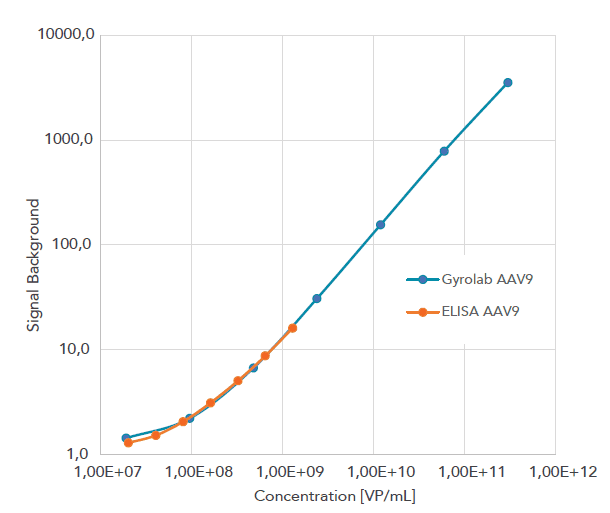
The Gyrolab miniaturized immunoassay technology offers a powerful automated alternative that generates data comparable to ELISA but with less hands-on time, lower consumption of precious sample and reagents, and minimal matrix interference. It also provides higher precision, and a broader dynamic range that reduces the need for re-analysis. The Gyrolab software supports 21 CFR Part 11 and the system is thereby compliant to be used in a regulated environment such as QC labs. The below example shows the increased dynamic range of a AAV9 titer assay on Gyrolab (blue line) as compared to manual ELISA (orange line).
This white paper provides an overview of the viral vector landscape and its use in cell and gene therapy, with focus on lentiviral vectors and adeno-associated viral (AAV) vectors. The requirements for immunoassays in viral vector bioprocess analytics are discussed in detail and Gyrolab case studies from viral vector analytical labs are presented. Find out how many labs are experiencing improved productivity when implementing the Gyrolab technology in their bioprocess analytical workflows.
Download the PDF now.






