Europe’s cell and gene therapy sector has stagnated but advocacy group the Alliance for Regenerative Medicine (ARM) hopes upcoming legislation can reinvigorate the sector.
In many areas of pharma, Europe has been at the forefront. The continent approved biosimilars, for example, nearly a decade before the US. And for cell and gene therapies (CGTs), the European Medicines Agency (EMA) has often given the green light ahead of their regulatory counterparts across the globe.
For example in 2022, Europe approved both Biomarin’s single-dose hemophilia A gene therapy Roctavian (valoctocogene roxaparvovec) and Atara’s Ebvallo (tabelecleucel), the world’s first allogeneic T-cell. Neither have been greenlighted across the pond.

Image: DepositPhotos/
g0d4ather
And according to an ARM industry snapshot, the first CRISPR therapy to treat a genetic disease – CRISPR Therapeutics and Vertex Pharmaceuticals’ sickle cell disease candidate exagamglogene autotemcel (exa-cel) – is likely to be approved in the EU this year.
Stagnation
But while the EMA is often a pioneer in approvals, the continent as a whole is suffering when it comes to elements of advanced therapy medicinal product (ATMP) funding and development.
“There are numerous signals that changes are needed in the EU’s ecosystem for ATMPs,” Paolo Morgese, head of Public Affairs, Europe, at the ARM tells this publication. “Clinical trials, investment, and the number of ATMP developers have stagnated while increasing substantially in North America and Asia-Pacific, particularly the US and China.”
As ARM’s data shows below, the number of developers and trials in Europe in 2022 were a fraction of the number in the US and Asia.
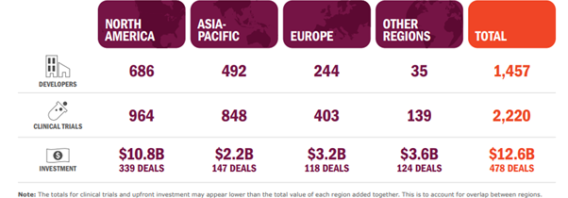
THE SECTOR IN 2022, taken from ARM’s Sector Snapshot, April 2023
Meanwhile, seven of the 25 ATMPs approved by the EMA have been withdrawn from the market for a variety of commercial reasons. Most infamously, Bluebird Bio withdrew its two approved gene therapies, Skysona and Zynteglo, from the market citing the untenable reimbursement model in the region.
Legislative approach
ARM and its members have been providing guidance to the European Commission on how legislation revision can create a forward-looking framework for ATMPs.
Specifically, the advocacy group is addressing the lack of harmonization surrounding the hospital exemption – a regulated pathway that allows the use of ATMPs within the EU under restrictive conditions overseen by national medicine agencies – and providing guidance on methodology for the new Joint Clinical Assessment (JCA), which aims to substitute parallel evaluations of clinical data conducted across multiple member states.
“There are clearly challenges related to many HTA [health technology assessment] processes, which were developed for traditional small molecule medicines and often require randomized control trials to establish benefit over the current standard of care,” says Morgese.
“These trials are often not feasible or ethical in the rare disease populations that many CGTs treat. That’s why the implementation of the new JCA process at the EU level is so important for the future of CGTs in Europe.”
According to Morgese, from 2025 ATMPs and cancer medicines will be the first therapies to go through this process, which could potentially reduce the need for each European Member States to conduct its own separate assessment. “However, if the new JCA simply adapts the most conservative approaches in use in some Member States, the evaluations of ATMPs will be inconclusive, impeding access to ATMPs.”
Morgese also speaks of ARM’s concerns over the Hospital Exemption, part of the regulation that established the ATMP framework.
“The intent of the exemption – which ARM supports – is to provide individual patients with high unmet medical needs for which there is no EMA-authorized treatment available to receive ATMPs created in hospitals. However, in practice Member States interpret the exemption differently and have a variety of regulatory standards under which they approve therapies, sometimes for larger patient populations than was intended by the regulation.
“Ultimately, this risks undermining the high regulatory standards of the EMA and creating two sets of rules – one for hospitals and one for developers. Developers want to see an even playing field and to have the certainty of high regulatory standards in order to keep investing in Europe. The EMA and patient groups have expressed similar concerns about lower regulatory standards.”

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)



schedl_b_and_w.jpg?width=400&auto=webp&quality=80&disable=upscale)


