Higher-Order Structure Comparability: Case Studies of Biosimilar Monoclonal Antibodies
https://bioprocessintl.com/wp-content/uploads/2014/06/062014_MichaelDavis.mp3
Great successes for monoclonal antibody (MAb)–based biologics over the past decade have provided many valuable options for patients combating some of the most serious diseases in the world, including cancer and autoimmune diseases. MAbs and antibody–drug conjugates (ADCs) are among the fastest growing biologic segments in development, with hundreds of candidates currently under clinical study.
Meanwhile, society is facing the challenge of increasingly higher costs in healthcare including the cost of pharmaceuticals. With an aging population in many parts of the world, striking a balance between providing an incentive for breakthrough medicine from innovator companies and controlling the high cost of medicines by means of national policy has motivated many governments to develop policies for approval of generic biologics. In 2012, the US Food and Drug Administration (FDA) released guidelines for development of biosimilars, paving the way to approval and marketing of such products on the largest biologics market in the world (1, 2). Even though many biosimilars have been marketed in other countries for years, reports show comparability challenges for many of those (3, 4).
Product Focus: Biosimilars
Process Focus: Manufacturing
Who Should Read: QA/QC, Product development, and analytical
Keywords: ELISAs, monoclonal antibodies, expression systems, immunogencity, characterization
Level: Intermediate
For successful development and marketing of biosimilars with desired efficacy and safety, this industry recognizes the central importance of extensive analysis comparing innovator and biosimilar molecules. It is also recognized in the biotechnology arena that our understanding of complex biologics remains limited even though we have many analytical technologies available to us. A recent FDA guideline for biosimilar development states the following:
The three-dimensional conformation of a protein is an important factor in its biological function. Proteins generally exhibit complex three-dimensional conformations (tertiary structure and, in some cases, quaternary structure) due to their large size and the rotational characteristics of protein alpha carbons. The resulting flexibility enables dynamic, but subtle, changes in protein conformation over time, some of which may be absolutely required for functional activity. . . At the same time, a protein's three-dimensional conformation can often be difficult to define precisely using current physiochemical analytical technology. (2)
With an understanding of our current capabilities in biologics higher-order structure (HOS) characterization, we developed an antibody array enzyme-linked immunosorbent assay (ELISA) to provide a new approach for evaluation of MAb HOS.
In a previous report, we showed that antibody arrays developed specifically toward marketed MAbs could detect structural differences that correlated well with other analytical readouts, including bioassays and glycosylation analysis (8). Experiments have shown that antibody arrays can detect subtle changes that sometimes were not detected by bioassays or any other analytical technologies currently available.
The arrays use more than 30 polyclonal antibodies to cover an entire MAb molecule, thereby measuring its surface-epitope distribution systematically and sensitively (Figure 1a
), whereas other assays measure only part of the molecule or give an average status of a biologic's population. So antibody array technology should be able to provide a unique measurement of biosimilar MAb HOS comparability. We suggest that additional surface exposure from a baseline readout be termed conformational impurity(8).
Figure 1a: Diagram of the antibody array enzyme-linked immunosorbent assay (ELISA)
Another advantage for antibody array technology is its ability to quantify small amounts of conformational impurity using an easy-to-operate ELISA format (Figure 1b
). As little as 0.1% conformational differences could be detected from all areas covered by the polyclonal antibodies, thus providing for accurate and sensitive measurement of the status of a MAb's conformation. No data yet correlate the impact of conformational impurity with efficacy and safety of a biosimilar MAb. But it is reasonable to postulate that more conformational impurities (epitope exposures) would increase the risk for potential immunogenicity if those additional epitopes were originally inside the innovator MAb molecule, which has been proven to be tolerated by patients’ immune surveillance systems. A significantly different new epitope exposure could break self-tolerance to a MAb and induce immunogenicity. Furthermore, increased exposure of new epitopes raises the possibility of a biosimilar MAb interacting with other regulatory proteins in a patient's body, causing off-target effects.
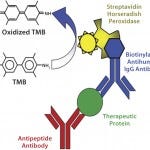
Figure 1b: ELISA format for the antibody array technology
Here we provide several case studies of biosimilar MAbs in development, furnishing a glimpse of the current biosimilar MAb development landscape.
Materials and Methods
Reagents: We purchased all chemicals from Sigma-Aldrich and 96-well microplates from Corning Inc. Streptavidin–HRP conjugate and biotin labeling kits came from Thermo Scientific.
Antibodies and ELISA Kits: All the antibodies and ELISA kits we used for this study are products of Array Bridge Inc. Polyclonal antibodies against the MAb peptides were produced by New Zealand white rabbits.
For the sandwich ELISA, antibodies against each region of the MAb molecule were coated on a 96-well plate, with each antibody coating six wells in rows B–G. In each column of the coated plates, we incubated the upper three wells (B–D) with a reference MAb (the marketed innovator MAb) in triplicate and the lower three wells (E–G) with a biosimilar MAb candidate in triplicate. We used a biotin-labeled rabbit antihuman IgG antibody to detect the MAb-peptide antibody complex and streptavidin-HRP to detect the complex formed by antihuman IgG-MAb–peptide antibody.
Signal strength for the sandwich ELISA depends on the MAb's relative epitope exposure in each region. The more epitopes from the MAb that can be recognized by the peptide-derived antibody, the stronger will be the signal produced. And the fewer recognized, the weaker the signal will be.
We used 3,3′,5,5′-tetramethylbenzidine (TMB) as a substrate for the HRP enzyme activity assay. Following a short development time to allow for color formation from the HRP enzymatic activity, we added an equal volume of 1M sulfuric acid to stop the reaction. Using a SpectraMax M3 spectrophotometer from Molecular Devices, we measured the color change at 450 nm.
Results
Case Study 1 — Molecules Showed High Conformational Similarity: In the first case study, we analyzed multiple batches of innovator Herceptin (trastuzumab) and biosimilar MAbs using the antibody-array ELISA. For all coverage areas measured using the 34 antibodies (data not shown), we detected no differences larger than the method variation (15% relative standard deviation, RSD). Because it has been shown that the antibody-array ELISA could detect ≥0.1% new-epitope exposure in a native MAb population, the results suggested that the biosimilar MAb has ≤0.1% conformational impurity compared with the original molecule.
It should be noted that this is a rather rare case in comparability studies. Most biosimilar MAbs we have tested show some conformational difference in certain areas of the molecular structure. So these are very interesting results. On one hand, it demonstrates that it is possible to develop a cell line to produce a biosimilar MAb that is highly similar to the innovator molecule in HOS. On the other hand, this is the only case we have encountered with no observed conformational differences according to the antibody-array ELISA. That underscores the difficulty of achieving complete conformational similarity between originator and biosimilar molecules.
Because this is a very sensitive method of detecting conformational impurity, the minor differences we detected may not affect the efficacy and safety of the product. However, it is still a valuable method that can characterize such conformational comparability — and more important, quantify the differences and help determine where conformational differences are introduced. With information provided by this conformational array ELISA, it sometimes may be easy then to improve a bioprocess to produce the biosimilar MAb with higher similarity in HOS to that of the innovator molecule.
Case Study 2 — Molecules Showed Good Similarity with Minor Differences in Several Parts of the MAb: Many biosimilar MAb molecules we have tested fall into this category. In this particular study, we compared Avastin and biosimilar bevacizumab molecules using the antibody-array ELISA. We found an increase of signal across the whole antibody panel (Figures 2a
and 2b
), which suggests that a small population of MAbs is unfolding. In addition, a few regions showed higher-than-average epitope exposure. That indicates additional epitope-exposure in those regions, and based on the spike testing of unfolded MAb, it represents 0.1%–0.2% new epitope exposure (8). Bioassays indicated no efficacy difference for the biosimilar molecule, so we considered this a minor deviation of the MAb HOS compared with the innovator molecule. Further development is under way for this biosimilar product.
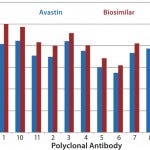
Figure 2a: HOS comparability of Avastin and biosimilar MAbs in the variable region
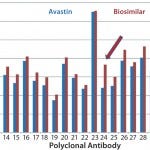
Figure 2b: HOS comparability of Avastin and biosimilar MAbs in the constant region
Case Study 3 — Biosimilar MAb Showed Good Similarity to Innovator, but Conformational Variations Were Detected Between Batches: This case compared a Humira (adalimumab) biosimilar candidate with the innovator molecule using three batches each. One batch of the biosimilar MAb matched very well with the marketed product in its conformational-impurity profile.
However, results from the other two batches of biosimilar adalimumab indicated an increased surface-epitope exposure across all regions covered by the polyclonal antibody array. That suggested a general unfolding of a small percentage of the MAb, representing a 0.1–0.2% new-epitope exposure. Considering that some biosimilars will be used at relatively high doses, a 0.2% new-epitope exposure could bring a significant number of structurally different molecules into development. The three batches of biosimilar adalimumab showed three different conformational impurity profiles, with one batch matching well with that of Humira (Figure 3).
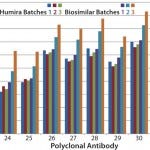
Figure 3: HOS comparability of Humira and biosimilar MAbs in the constant region-2
As mentioned above, MAb unfolding can raise the risk of immunogenicity by presenting new epitopes that usually are buried inside a MAb molecule on its surface. That increases the risk of breaking the tolerance of a patient's immune system (5, 6, 7). In this case study, the challenge appeared to be in process control because at least one batch of biosimilar adalimumab matched well with the innovator molecule. Further testing may show that the generally small level of MAb unfolding was introduced in purification or formulation processes, or it may be a packaging or storage issue.
Case Study 4 — Biosimilar MAb Showed Significant Conformational Differences: Several biosimilar MAb molecules we have tested fall into this category. In one instance, the biosimilar developer noticed changes in MAb stability. Bioassay testing showed that the biosimilar MAb lost potency in an ELISA-based binding assay; however, no analytical testing used could detect structural changes. With the antibody-array ELISA, the company found new epitope exposures in several areas of the molecule (data not shown). Some of those areas are close to “hot spots” of conformational variability, such as the hinge region and the area around the glycosylation site.
In another study, we tested Herceptin and a corresponding biosimilar molecule for HOS comparability and detected significant changes (Figures 4a
– c
). Based on spike testing (8), the increase in new-epitope exposure could represent from 0.5% to as high as 5.0% of the MAb population. Because the antibody-array ELISA signal is not all linear — especially with epitope exposure >0.5% — an accurate estimate cannot be made for each region. If we assume that some regions have 5% new-epitope exposure and that the dosage for this biosimilar is 10 mg/kg, for a 70-kg patient, fully 35 mg of MAb could be received with significant new-epitope exposure. That could well bring an increased risk for immunogenicity and other off-target molecular interactions.
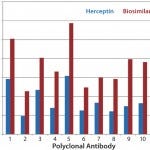
Figure 4a: HOS comparability of Herceptin and biosimilar MAbs in the variable region
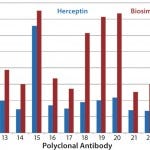
Figure 4b: HOS comparability of Herceptin and biosimilar MAbs in the constant region-1
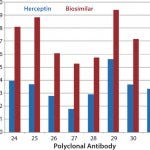
Figure 4c: HOS comparability of Herceptin and biosimilar MAbs in the constant region-2
When conformational analysis indicates significant changes, the next step is to investigate where in the development process those changes were introduced. For example, biosimilar MAb from cell-culture supernatant could be compared with an innovator molecule provided that no significant MAb unfolding is present. (Because the principle of the antibody-array ELISA is to detect new-epitope exposure, unfolded MAb will expose many epitopes, producing a stronger assay signal and complicating detection of regional changes.) If that test indicates similarity in conformational profile between the biosimilar MAb from supernatant with the innovator molecule, then the conformational differences probably were introduced during downstream processing, formulation, or storage. Using the antibody-array ELISA, that bioprocess could be further optimized.
If, however, a MAb from cell-culture supernatant shows significant conformational differences compared with the innovator product, then those differences probably were introduced in cell-line development and/or the culture process. In such a case, new cell lines need to be screened from the original transfection pool. This can be a costly action if a project has been in development for years, and it takes significant time and effort to develop a new cell line.
Therefore, the best time to use an antibody-array ELISA in biosimilar MAb development is at the cell-line development stage. There, the option exists to evaluate multiple cell lines for critical attributes, including conformational impurity/HOS profile, at a reasonable cost.
Because this ELISA measures the conformational status of a biosimilar MAb, information obtained from such an assay overlaps with many other analytical measurements: bioassays and tests of degradation, oxidation, aggregation, and glycosylation. Thus, the antibody-array ELISA could be considered a measurement that reflects many critical aspects of a biosimilar MAb in development. However, in this particular case study, the real value of this assay came in its measurement of conformational impurity, which no other analytical technology could systematically and sensitively measure.
Case Study 5 — Innovator Molecule Also Could Have Process Deviation: In this case (data not shown), we analyzed many batches of a best-selling MAb from both the European and US markets using the antibody-array ELISA. Results showed that all batches from Europe had consistent conformational profiles; however, results from several batches of the MAb on the US market indicated that one batch showed significant conformational differences in a few MAb regions. Representing 0.1–0.2% new-epitope exposure for that particular batch, those differences may or may not have had an effect on product efficacy or safety.
Almost all biologics currently on the market have immunogenicity issues to a certain degree (5), but it is not known whether this particular conformational impurity contributed to the MAb's immunogenicity. A market follow-up with that specific batch would help answer that question.
The fact that this product batch was released to market based on the manufacturer's own release-testing suggested that the company's current analytical testing was unable to detect complex structural differences that could prove to be important for efficacy and safety of its marketed biologics. That reflects a need to further develop analytical technologies for more comprehensive MAb characterization. However, if it is shown that those conformational deviations do not affect product safety and efficacy, then this analysis would provide a benchmark for biosimilar MAb development and eventual market release.
Case Study 6 — Biosimilar MAb Produced from Plant Cells: Among the many biosimilar MAbs we tested, one was made using plant cells (data not shown). We found significant conformational differences to be present for the biosimilar molecule, with many regions showing >1% new-epitope exposure. No data from other analytical analysis are available for this particular molecule, but the difference we observed from our conformational-comparability analysis presents a concern because of its magnitude and extent.
This is the only case of conformational testing for a MAb derived from cell lines other than Chinese hamster ovary (CHO) or nonsecreting-null murine myeloma (NS0) cells. And it is unclear whether the plant cell line or the purification process — or both — contributed to these conformational impurities. It will be interesting to test more MAbs derived from plant or other cell lines to determine whether the expression hosts will significantly affect the MAb conformational status.
One thing is clear, however: Biosimilar MAbs from different developers showed a wide range of conformational profiles even when all the molecules tested came from CHO cells. So not all CHO cells are created equal — and/or the specific innovator processes are not duplicated as easily as biosimilar developers hoped.
Discussion
Antibody-array technology was developed to systematically measure epitope exposure and compare conformational status of biosimilar MAbs and their corresponding innovator biologics. During the past two years, we have tested many biosimilar MAbs at different development stages from several countries, with interesting findings. A few biosimilar developers have been able to produce MAbs that are highly similar to the reference molecules, whereas other molecules have showed different degrees of HOS variance. Some of those differences correlated with bioassay or other structural characterization results, and antibody-array technology also provided regional structural information that sometimes other technologies could not provide. Because of the large panel of antibodies covering each whole MAb molecule, the surface epitope distribution measured by an antibody-array ELISA could help biosimilar developers select cell lines to produce MAbs with the best-matched critical attributes compared with the innovator molecule, including its HOS. In addition, antibody-array technology could be used in downstream process optimization and formulation development.
Author Details
Corresponding author Xing Wang is president, Qing Li is a scientist, and Michael Davies is a senior scientist at Array Bridge Inc., 4320 Forest Park Avenue, Suite 303, St. Louis, MO 63108; 1-636-284-4212, fax 1-314-932-4038; [email protected]; www.arraybridge.com.
References
CBER/CDER. Feb 2012. Guidance for Industry: Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. US Food and Drug Administration, Rockville, MD www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf
CBER/CDER. Feb 2012. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. US Food and Drug Administration, Rockville, MD www.basinc.com/services/gen/BiosimilarsScientific.pdf
Parnham, MJ, Schindt-Horvat, J, and Kozlovic, M. 2007. Non-Clinical Safety Studies on Biosimilar Recombinant Human Erythropoietin. Bas. Clin. Pharmacol. Toxicol. 100:73-83.
Schellekens, H.2009. Assessing the Bioequivalence of Biosimilars. Drug Discov. Today. 14(9):495-499
Jiskoot, W.2009. Immunological Risk of Injectable Drug Delivery Systems. Pharmaceut. Res.26(6):1303-1314
Hermeling, S. 2005. Structural Characterization and Immunogenicity in Wild-Type and Immune Tolerant Mice of Degraded Recombinant Human Interferon Alpha 2b. Pharmaceut. Res. 22(12):1997-2006
Hermeling, S. 2004. Structure–Immunogenicity Relationships of Therapeutic Proteins. Pharmaceut. Res. 21(6):897-903
Wang, X., Li, Q, and Davies, M. 2013. Development of Antibody Arrays for Monoclonal Antibody Higher Order Structure Analysis. Front. Pharmacol. 4:103 www.ncbi.nlm.nih.gov/pmc/articles/PMC3748713
You May Also Like





