
Figure 1: Overview of timeline from proof of concept to single-use prototype
The production of increasingly higher cell densities has stressed the already limited solids-handling capabilities for traditional intermittent ejection centrifuge systems. By contrast, a single-use disc-stack centrifuge based on the solids-flow principle offers distinct advantages for cell culture harvesting. Such benefits include solids handling of high-density cell culture processes and elimination of the separation disruption and aerosol generation associated with the intermittent solids ejection. A single-use system also provides well-established benefits of disposable components — such as removal of steam- and clean-in-place (SIP/CIP) operations — while enabling a fully closed centrifuge system through the elimination of a discharge mechanism.
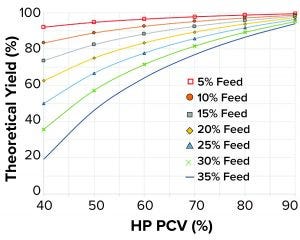
Figure 2: Theoretical yields as function of feed packed cell volume (PCV) and heavy-phase (HP) PCV
Design Approach
To succeed with a fully contained continuous solids-transportation system, a concentrated cell suspension must be forced to move against the system’s g-force from the solids-collection area. Two parameters key to achieving that are the cells’ interacting properties and the rheological behavior of a thick cell suspension when shear forces are applied.
Cell-interaction properties describe how cells stack together when concentrated and compacted in a g-force field, here denominated as angle of repose (1). A steep angle of repose means that the angles of cells compacted inside a rotating system also are steep. That will influence the choice of conical discs, angles applied inside the solids space, and the ability of solids to move along a bowl’s periphery.
When a cell suspension flows through a rotating system, the heavy phase (HP) is subjected to shear forces. If the suspension has shear thinning properties, then the viscosity of a suspension decreases with applied shear and thus becomes easier to transport out as a liquid-like phase (2). Once outside the influence of those shear forces, the suspension reverts to its original viscosity.
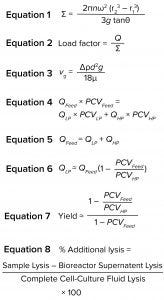 Because no synthetic particle with the same properties as Chinese hamster ovary (CHO) cells has been identified, our development work has been performed using cell culture fluid. The equivalent settling area of a disc-stack centrifuge, sigma value Σ (m2), can be expressed as Equation 1 (3). In that equation, n is the number of discs, θ is the disc’s half-cone angle (degrees), r1 and r2 (meters) are the respective inner and outer radii of the discs, and ω (radians/second) is the angular rotation frequency of the disc-stack centrifuge.
Because no synthetic particle with the same properties as Chinese hamster ovary (CHO) cells has been identified, our development work has been performed using cell culture fluid. The equivalent settling area of a disc-stack centrifuge, sigma value Σ (m2), can be expressed as Equation 1 (3). In that equation, n is the number of discs, θ is the disc’s half-cone angle (degrees), r1 and r2 (meters) are the respective inner and outer radii of the discs, and ω (radians/second) is the angular rotation frequency of the disc-stack centrifuge.
The volume of feed material through a centrifuge device per second is Q (m3/s). When Q is divided by the Σ value of the centrifuge, the result is the load factor (Equation 2). That factor can be used for scaling to flow rates in other sizes of similarly designed centrifuges (with a different area equivalent).
Equation 3 is known as Stokes law. In that equation, vg (m/s) is the Stokes’ settling velocity of a single solids particle in the gravitational force field, d (m) is the particle diameter, Δρ (kg/m3) is the difference in density between the solids particle and the suspension liquid phase, and μ (kg/ms) is the Newtonian dynamic viscosity of the liquid phase. A particle half the original size, for example, would result in a settling speed that is one quarter of the original value. To compensate for that, the residence time must be increased four times to achieve the same light phase (LP) liquid clarity. Doing so for a continuous process would require decreasing the flow rate or load factor to one quarter of the original value. Shear-sensitive solids need gentle treatment to minimize the formation of debris particles.
Separation is conducted volumetrically. The amount of solids entering with the feed media must be removed through the HP solids outlet. By determining the amount of cell material in the feed and choosing a concentration of solids in the HP, the amount needed to be removed through the LP and HP are calculated with Equations 4 and 5, in which PCV is the packed cell volume.
The value of PCVLP approaches zero after material passes through a centrifuge (Equation 6). If yield is defined as the amount of recovered product in the LP compared with the total amount in a cell culture fluid, a theoretical yield can be calculated using Equation 7. If we assume that the concentration of a product is the same in the cell culture fluid (cell material excluded) as it is in the LP liquid, then the theoretical yield can be calculated according to Equation 7.
When plotting theoretical yield according to Equation 7 as a function of the feed PCV from 5% v/v up to 35% v/v and the HP PCV from 40% v/v up to 90% v/v, yields >90% are achieved easily in normal fed-batch feed concentrations. Such yields can be achieved even when running fairly dilute HP such as HP PCV from 40% v/v to 50% v/v (Figure 2). Conversely, when separating cell culture fluids containing high feed PCV, (>25% v/v), reaching a 90% yield would demand an HP PCV >77% v/v. In general, an increased amount of solids present in a feed material requires higher HP PCVs to achieve high yields. HP PCVs as high as 97% v/v have been achieved in such tests, but for very high concentrations, lowered LP clarity has been observed.
Methods
Feed PCV is a sample of a homogeneous suspension of cell culture fluid that is spun down in a centrifuge in a volumetric tube with graduated markings. We used this to approximate solids volume percent content of samples at 830g for 10 minutes.
HP PCV samples are spun down at high g-force in a centrifuge with volumetric tubes having graduated markings appropriate for samples with high solids samples. We used these samples to approximate solids volume percent content of test samples at 12,000g for five minutes.
Yield was derived from a mass balance. We compared grams of protein per treated volume from the starting cell culture fluid with those from the recovered material. Yield is calculated with an adjustment for cell volume from the PCV of the starting cell culture fluid and provided as a percentage.
Filtration Throughput: A filter screening system processed LP at a constant flux of 300 L/m2/h (LMH) through a two-stage filtration train. The area ratio between the two stages was about 1:1. The first stage was a dual-layer depth filter with pore-size ratings of 3–0.1 µm. The second stage was a dual-layer membrane filter with a 0.2-µm rating. The system monitored differential pressure continuously between each stage, and a total system pressure of 30 psi defined the endpoint. Filtration throughputs were the volumes achieved per given area of the first-stage depth filter at a system pressure of 30 psi with no safety margin.
Lysis was measured by looking for plasma-membrane rupture, which was quantified by measuring the release of intracellular proteins (4, 5). For this study, we assayed the cell culture material based on the content of lactate dehydrogenase (LDH) in the samples. A 100% lysed sample was determined using saponin as a lysing agent and comparing it with samples of the clarified material, with the result given as a percentage. Additional lysis was calculated by using Equation 8.
Viability is measured with an instrument that captures images from a hemocytometer and compares the number of live and dead cells as distinguished by Trypan blue dye.
Turbidity was measured off-line on LP samples using a Hach 2100N turbidimeter, with a measurement range of 0–4,000 nephelometric turbidity units (NTU).

Figure 3: Overview of the three evaluated configurations in the proof-of-concept test
Test Overview
Proof of Concept: The first experiment, performed in early 2018, was a proof-of-concept test to determine whether the continuous solids removal method provided acceptable yield (>90%), lysis (<15% additional), and filtration throughput (>300-L/m2 capacity with filters selected) as compared with results from a solids-ejecting centrifuge. We performed these tests using a stainless-steel pilot-test centrifuge (Alfa Laval Explorer) and challenged with a cell culture fluid with 6.6% PCV. We evaluated three configurations focused on solids handling within the bowl to enable the elimination of the discharge method for solids removal, which has been an engineering challenge in the design of a single-use centrifuge (Figure 3).
Configuration 1 included a top disc that extended into the solids-collection space. This design allowed the solids flow to be directed out of the bowl across the entire surface of the top disc. The design is identical to that of the Bactofuge separator used by Alfa Laval for stainless-steel centrifuges.
Configuration 2 included a top disc with two small tubes for HP flow removal. The intent for this design was to increase velocity of HP flow compared with that of Configuration 1.
Configuration 3 was a modified version of Configuration 2 with a single tube for HP flow.
For performance comparison in this experiment, we used a stainless-steel, intermittently solids-discharging Alfa Laval Culturefuge 100 (CF100) centrifuge. The variable parameters included bowl speed, load factor, and split ratio between light and heavy phases. The split between phases when operating test configurations 1–3 was controlled manually using regulating diaphragm valves. For all tests, feed was supplied by a peristaltic Watson-Marlow 600 series pump. We set the load factor from the reference test in the CF100 centrifuge as the initial benchmark for our proof-of-concept tests.
High-Density and Stress Testing: We performed the second development test again using the stainless-steel Alfa Laval Explorer pilot-test centrifuge. The bowl speed was reduced by 13% compared with the proof-of-concept tests to better represent a future single-use designed bowl. We converted process components and process lines into single-use components for this test. The solids transportation method selected used a modified configuration 2 design from the proof-of-concept test with a single HP tube.
Our primary purpose for this experiment was to evaluate the suitability of a chosen configuration when operating with a high PCV feed stock. Feed material was an analog made by combining cell culture fluid with centrifuged HP to achieve a PCV of 28%. The secondary goal for this experiment was to prove that a solids flow once paused could be restarted. We tested that by mimicking a process interruption for a set period and then resumed the process flow.
Tuning of Internal Design for Improved Performance: For the third development test, we used a cell culture fluid with 7.3% PCV. The study was performed on a full single-use prototype system in which the rotating process wetted parts consisted of polymeric injection molded or three-dimensional (3D) printed material. We used a centrifugal single-use feed pump instead of a traditional peristaltic type. The centrifugal pump was selected to both eliminate pulsation interference and safeguard the system for higher pressure spikes that can occur with positive-displacement–style pumps.
The main objective was to evaluate the effect of subtle variances inside the rotating system. They included size, length, and shape of feed distribution zones in the inlet; design of the caulks (spacers giving distances between each disc); and size and type of the solids-phase outlet. We evaluated six different configurations that are not detailed herein. Our goal was to maintain or improve the performance previously observed while still achieving a thick solids suspension and maintain a high yield and filter throughput with low turbidity and additional lysis.
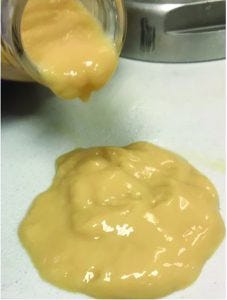
Figure 4: Thick solids suspension from heavy phase

Figure 5: Cleaned light phase
Results
Proof-of-Concept Experiment: Figure 4 shows a solids-suspension sample that was collected from the continuous HP outlet. The PCV in the HP sample was determined to be ~70% when further compacted at high g-force in a laboratory centrifuge at 12,000g. Maximum achieved thickness during the test was 80% v/v.
While a thick HP suspension was being produced, turbidity of the LP decreased when compared with that of the CF100 sample (Figure 5). So the concept holds at least in terms of separation efficiency and continuous solids transportation when operating with a feed concentration of 6.6% PCV.
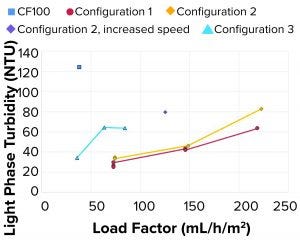
Figure 6: Light-phase turbidity at different test conditions
When initially benchmarking at the same flow rate — the LP turbidity from configuration 1 at 73 mL/h/m2 as compared with that of the CF100 control sample at 38 mL/h/m2 — we demonstrated that configuration 1 produced clearer LP. The flow rate and load factor could be increased further while we maintained acceptable sample clarity. One reason is that the single-use proof-of-concept configuration operated at a lower bowl speed, thus causing less shear and lower lysis of the cells during rotational acceleration. Figure 6 shows that when bowl speed was increased in one of the test points (HP tubes, increasing speed), the turbidity of the LP roughly doubled because more residual solids left the system, even though the area equivalence had increased (increased bowl speed and decreased load factor).
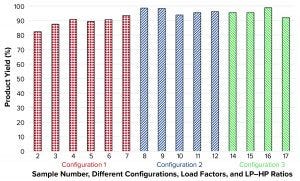
Figure 7: Product yield
Figure 7 shows that calculated yields were high for all three tested configurations independent of evaluated flow rates. Those values are influenced by how aggressive the split ratio was set. In initial samples 2, 3, and 5, the volumetric split of the incoming feed was set to 80%/20% and 85%/15% between LP and HP. So the corresponding HPs became slightly diluted, and excessive product was lost.
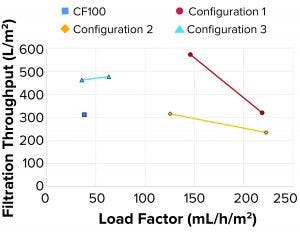
Figure 8: Filterability
Product Yield: Those samples all had yields <90%. When increasing the volumetric split to 90%/10% or even 93%/7%, as is the case for other samples, all yields were >90% independent of tested load factor and flow rate. No yield data could be presented for sample 1 (CF100 reference) because the feed and LP volumes during sampling were omitted. The other missing sample in Figure 7 (sample 13) is not a process setting, but rather a new reference sample taken from cell culture media in the bioreactor.
Filterability tests performed on a subset of samples indicated that large filtration volumes could be processed through both the depth and sterile membrane filters. All the tested configurations (Figure 8) achieved the same or larger filtration throughput compared with the reference sample from the CF100 sample.
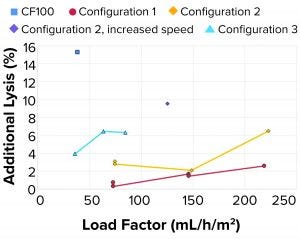
Figure 9: Additional lysis
Filterability: Filtration throughput is a function of the quantity and composition of solids remaining in the LP. As the load factor increases, an increase in solids present in the LP is expected because of the shorter residence time inside the rotating system. The split ratio (how much is diverted to LP and HP, respectively) also is determinant of filtration throughput. As the volume directed to HP reduces, the sample becomes thicker, and less liquid is lost. A drawback, however, is that some of the solids are forced into the LP. Consequently, a lower filter throughput is achieved before reaching maximum pressure.
As observed in the previous case, the additional lysis (Figure 9) caused by rotational acceleration and pressure drop was lower than both the reference centrifuge and the targeted metric (<15% lysis) in all tested configurations. At higher load factors, cell velocities within the system increase, which can increase cell shear.
Additional Lysis: It should be noted that cell lysis as measured through LDH concentrations in LP is generated upstream of the point for cell separation from continuous liquid (feed line, feed pump, and rotational acceleration at inlet of centrifuge) and in the internal feed-distribution zones before the disc stack.
Lysis of separated cells also can occur during transport through the HP flow path. As measured through LDH concentrations, the degree of lysis depends on the amount of shear the cells are exposed to. That will be observed in only HP because cell separation in that case already has occurred. When increasing the rotational speed of the centrifuge bowl by 25% for configuration 2, lysis in the inlet increases three to four times.
The proof-of-concept experiments showed that continuous flow of a thick suspension of CHO cells was possible while maintaining a low turbidity and high filterability of the LP. The resultant high yield values as well as decreased lysis of the cells in the feed line and rotating bowl also were positive results.
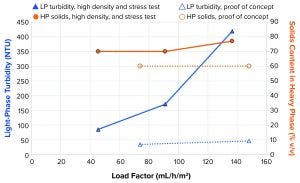
Figure 10: Light-phase turbidity and heavy-phase concentration at different load factors
High-Density and Stress Testing: In the proof-of-concept case (Figure 6 and dotted lines in Figure 10), 34 NTU and 46 NTU could be achieved at load factor values of 74 mL/h/m2 and 148 mL/h/m2, respectively, when operating in configuration 2 with a feed PCV of 6.6%. For that test, when the system operated with 28% v/v feed analog material, turbidity of the LP reached 85 NTU, 171 NTU, and 419 NTU at 46 mL/h/m2, 91 mL/h/m2, and 137 mL/h/m2, respectively. Operation on high solids content in the feed with good clarity of the LP still was possible with that operational principle. Turbidity was considerably higher than in the initial proof-of-concept test when we compared similar load factors.
Figure 10 also shows that volumetric setpoint of the solids in the HP for the “high density and stress test” was 70%. In two of the three high-density samples (load factors of 46, 91, and 137 mL/h/m2, Figure 10), the measured HP PCV matched the setpoint, but the third was slightly higher at 77% v/v. The measured volumetric solids content of the HP proof-of-concept sample was lower at 60%. That made the separation task slightly easier with the lower HP PCV. The main contributor to the higher turbidity is probably the much higher concentration of solids entering the separation space. That was measured at more than four times higher cell concentration in the high-density test than in the proof-of-concept test.
Another complexity with LP turbidity is cell viability in a bioreactor. Low viability can indicate the presence of dead cells, which are prone to shear, increasing the cellular debris that are more difficult to separate. When we compared results from the proof-of-concept test and the high-density and stress test, we found that viability was 83.8% and 97%, respectively. Consequently, viability should act in favor of the latter test in terms of turbidity in the LP.
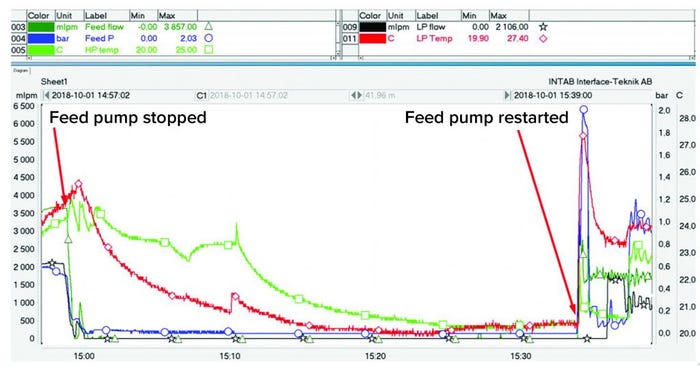
Figure 11: Stress test of a process
To evaluate system robustness, we introduced a process interruption after three hours of centrifuge operation, stopping the process feed to the unit. During the interruption, the centrifuge bowl continued spinning at its normal speed to compact the cells in the periphery further. After 35 minutes, the feed pump was restarted (Figure 11), and the feed pressure increased to 2 bar before flow resumed in both HP and LP outlets. Once flow was reestablished, the separation process continued as expected.
Temperature peaks of LP and HP, measured in the outlet tubing, also could be identified when the flow was reestablished after the compaction test. The increase in temperature was caused by air friction heating the rotating bowl and its contents while the flow was interrupted. Once this material was displaced from the bowl, temperatures of both phases recovered to the previous values.
Results show that operation on high-feed PCV is possible with the chosen design principles. A cell culture batch or a single-use process consumable is not lost because of a processing pause. When operating with CHO cells, the process can be resumed even after long compaction by g-force.
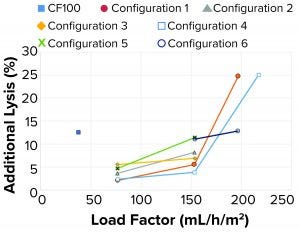
Figure 12: Additional lysis at different configurations and load factors
Tuning of Internal Design for Improved Performance: For this portion of the test plan, we took all samples with an HP setpoint of 70% v/v. At low flow rates or load factors, the additional lysis of the feed material entering and passing through the separation device was between 2% and 6% for all tested configurations detailed above (Figure 12). When the load factor was doubled, additional lysis also increased. For configurations 1, 3, and 4, that increase is moderate with additional lysis between 4% and 7%. For configurations 2 and 5, the increase is substantial with additional lysis between 8% and 11%. When further increasing the load factor (flow rate) to about three times the original ratio, the additional lysis increased to 25%.
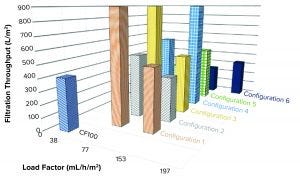
Figure 13: Filtration throughput at different configurations and load factors
A more detailed review revealed the main cause for increased additional lysis to be related to centrifugal pump speed. At medium feed flows and load factors, pump speed ranged between 4,600 rpm and 6,100 rpm. At a high load factor (153 mL/h/m2), pump speed varied from 6,200 rpm to 7,000 rpm, with moderate lysis present. The highest lysis was observed when tripling the load factor (197–217 mL/h/m2) for configurations 1 and 4. In those samples, pump speed was 9,000 rpm (maximum speed), causing high additional lysis.
When the single-use prototype ran on medium to high load factors, 77–153 mL/h/m2, the amount of additional lysis was below the reference centrifuge value of 12.5% for the cell culture fluid.
The additional lysis data also correlated well with off-line turbidity measurements. All samples with low turbidity had low additional lysis, whereas samples with higher turbidity had more lysis.
When we filtered the LP sample from the reference centrifuge through a depth filter and a 0.2-µm membrane filter, we achieved a flux of 407 L/m2 before pressure above the target maximum 30 psi (pmax) was reached (Figure 13).
Samples taken from the different single-use prototype tests could achieve a throughput between 240 L/m2 and over 900 L/m2, depending on tested settings.
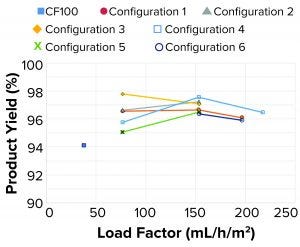
Figure 14: Product yields
As Figure 14 shows, all product yields were >90%. Because of the continuous solids-suspension removal method with a reduced product loss (compared with a typical intermittent solids discharge method in the reference centrifuge), high yields can be achieved when running the single-use prototype, independent of tested configuration.
With all tested single-use configurations, it is possible to achieve low additional lysis of the cells in the feed, with a large filtration throughput and high yield. Large filtration throughputs were achieved with some configurations when operating at low to medium load factors and flow rates. Excessive speed of the centrifugal feed pump caused a large increase in the additional lysis and must be avoided.
An Efficient Single-Use Harvest Method
The shear-thinning properties of CHO cell solids enable the elimination of traditional centrifuge solids discharge and facilitates the design of a new centrifugal single-use harvesting technique. Successful harvesting with high yield, high filterability, and low additional lysis has been achieved with different CHO-cell culture fluids and feed PCVs ranging from 6.6% to 28% and feed flows ranging from 1 L/min to 3 L/min. Yields of target molecules up to 98% have been achieved. Amounts of additional lysis were below the targeted 15%, thus demonstrating a gentle processing technique. Filtration throughput up to 900 L/m2 also was achieved. The extent of filterability depends on feed-flow rate, existing amount of debris in a bioreactor, and the desired HP PCV — thus a trade-off between harvesting time, filterability, and yield. Our study also showed that cell culture fluid and single-use consumables are not lost after a 30-minute process interruption. The process can be resumed by applying an extra amount of feed pressure.
Acknowledgments
The authors thank the following people for their contributions: Robert Barloga, Jerome Bill Jr., Håkan Björk, Joshua Borrajo, Luis Caldera, Kartoa Chow, Jeff Davis, Chris Dowd, James Dvornicky, Anders Ekström, Vidal Elzam, Robert Geiding, Ken Hamilton, Lars Hillström, Kasper Höglund, Roland Isaksson, Mike Laird, Per-Gustaf Larsson, Pär Leander, Debbie O’Connor, Doug Osman, Christian Randecker, Joseph Sexton, Amy Shen, Anders Stafrén, Vinson Tan, Peter Thorwid, Trisha Ray-Chaudhuri, Ganesh Vissvesvaran, Richard Weeks, Shirley Yip, and Jackie Yu.
References
1 Häggmark C, Königsson S. Rheological Characterization of Solids Phase in Biomass Processes of Disc Stack Centrifuges. Chem. Eng. Technol. 41(12) 2018: 2289–2297; https://doi.org/10.1002/ceat.201800288.
2 Iordan A, Duperray A, Verdier C. Fractal Approach to the Rheology of Concentrated Cell Suspensions. Phys. Rev. E 77(1) 2008: 011911; https://doi.org/10.1103/PhysRevE.77.011911.
3 Ambler CM. The Theory of Scaling Up Laboratory Data for the Sedimentation Type Centrifuge J. Biochem. Microbiol. Technol. Eng. 1(2) 1959: 185–205; https://doi.org/10.1002/jbmte.390010206.
4 Babson AL, Babson SR. Kinetic Colorimetric Measurement of Serum Lactate Dehydrogenase Activity. Clin. Chem. 19(7) 1973: 766–769; https://doi.org/10.1093/clinchem/19.7.766.
5 Kepp O, et al. Cell Death Assays for Drug Discovery. Nat. Rev. Drug Discov. 10(3) 2011: 221–237; https://doi.org/10.1038/nrd3373.
Ryan Hundley is senior technical specialist at Genentech; [email protected]. Staffan Königsson is process application development manager at Alfa Laval; [email protected].




















