First Biomass Monitor Integrated into Applikon Biotechnology's Bioreactor Control System


Applikon Biotechnology BV (in the Netherlands) and Aber Instruments Ltd. (in the United Kingdom) have entered into an agreement for the exclusive supply of the first integrated bioreactor controller and sensor for viable cells on the market. Applikon Biotechnology will offer the viable cell sensor and controller option in its EZ Control bioprocess controller portfolio designed for the biotechnological and biopharmaceutical industries.
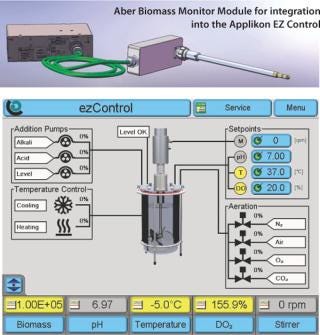
The Aber Biomass Monitor uses a unique in situ probe that incorporates four annular ring electrodes to apply a radio frequency field to the biomass. Electronic processing of the resulting signal produces an output which is an accurate measurement of the concentration of viable cells. The system is responsive to cells with intact cell membranes and is insensitive to gas bubbles, cell debris and cells with leaky membranes.
Aber Instruments, celebrating its 20th anniversary in 2008, has upheld its tradition of innovation and staying ahead of the competition by supplying clients in the biopharma, biofuel and brewing industries with a unique range of solutions. These have included steam sterilizable probes suitable for cGMP, a choice of five different instruments (including one instrument that also works off-line) optimized for different process applications or scale and a frequency scanning package that can be used to monitor critical process events in cell culture and high density microbial fermentations. The latest innovation, a module for integration into the Applikon EZ Controller, has been designed to meet GAMP4 requirements.
Applikon is the first company worldwide to offer a system that accurately measures and controls not only the standard set of process parameters (pH, temperature, dissolved oxygen, foam/level, and agitation) but also viable biomass in a single fully integrated solution. BioXpert SCADA software further enhances the capabilities of this system by offering 21 CFR 11 support, allowing the system to be operated in a validated cGMP environment.
Arthur Oudshoorn (managing director of Applikon Biotechnology) said, “This agreement is a further essential milestone in exploiting the full potential of novel technologies in measurement and control. In the past years, Applikon Biotechnology has built up a broad portfolio of unique bioprocessing devices for both cell culture and microbial cultivation, and this has considerably set us apart from the competition. We now are definitely a step ahead.”
Dr. John Carvell, sales and marketing director of Aber Instruments, explained the benefits of the agreement: “Applikon Biotechnology offers us the opportunity to partner with the market leader in laboratory-scale bioreactor systems for cell culture. In many of the key markets including North America, a talented distribution network is already in place to sell both Aber Instruments and Applikon Biotechnology so the customers will benefit from expert advice, guidance, and support for both product ranges. This agreement reinforces our tradition of innovation and meeting our customers’ needs.”
You May Also Like





