Biosimilars, Oxidative Damage, and Unwanted Immunogenicity
June 1, 2013
Concerns about the economic viability of biosimilars center on their high development cost relative to small-molecule generics, along with (and partly because of) the difficulty in demonstrating bioequivalence for these complex molecules. Immunogenicity is a particular area of increasing vigilance at both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (1, 2). Unwanted immunogenicity is an underlying cause of multiple deleterious effects for all protein-based therapeutics — including loss of efficacy, altered pharmacokinetics, and reduced stability (3,4,5,6,7,8) — and it poses a major risk for product failures and recalls.
Failure to demonstrate equivalent or (ideally) lower immunogenicity for a biosimilar is both costly and risky. Development of an unsatisfactory or inconsistent immunogenicity profile during development — or much worse, during postmarket surveillance — may be economically disastrous. It can even lead to costly reformulation work and additional clinical studies. Complicating the problem further, immunogenicity may sometimes arise only after repeated administration over an extended period. So it is imperative that all potential sources of unwanted immunogenicity be dealt with stringently as part of a risk-management plan during development to ensure a predictable, stable, and acceptable immunogenicity profile during manufacturing and storage through lot expiry.

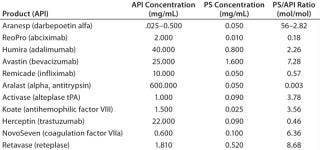
In final drug formulations, aggregation is dealt with largely by including surfactants. But other factors need to be considered during formulation optimization: pH, ionic strength, counterions, cosolvents, chelating agents, antioxidants, and antimicrobial preservatives. Tween 80 and Tween 20 polysorbates (PS-80 and PS-20, respectively) from ICI Americas are perhaps the most commonly used surfactants used in biotherapeutic formulations to prevent protein aggregation. Table 1 lists some such products containing them.
Polysorbates are effective in preventing protein aggregation, but they contain ether linkages (polyoxyethylene moieties). PS-80 has unsaturated alkyl chains that spontaneously and rapidly autooxidize in aqueous solution to yield protein-damaging peroxides, epoxy acids, and reactive aldehydes. Formaldehyde and acetaldehyde have been shown to induce unwanted immunogenicity in proteins, and in some instances they actually cause reaggregation of protein therapeutics (10,11,12,13,14,15,16,17,18,19,20). Those reactive species are continuously produced during manufacturing processes and throughout the time that biotherapeutics sit in inventory awaiting use, so the associated damage (and resulting immunogenicity) can progressively worsen.
At a May 2012 FDA public hearing on biosimilars, Richard Dolinar, MD — head of the Alliance for Safe Biologic Medicines (www.safebiologics.org), an organization of biotechnology companies, patients, and physicians — succinctly summarized immunogenicity concerns. “Unwanted immunogenicity is the preeminent safety challenge associated with all biological therapeutics and can result in unexpected and sometimes severe adverse effects. Complicating matters, side-effects may only appear in patients after higher doses or prolonged duration of treatments and may be attributed to a number of patient-, disease-, or product-related factors.” His full testimony is online at www.safebiologics.org/pdf/FDA/ASBM-Testimony.pdf.
Because immunogenicity of biotherapeutics is an important focus of concern by regulatory agencies, physicians, and informed patients alike, the need for nonautooxidizing replacement surfactants has become acutely apparent. This needs to be proactively addressed by the industry and its suppliers.
Immunogenicity Affects All Biotherapeutics
Induction of unwanted immunogenicity is among the most serious problems that result from protein aggregation (21). Such immunogenic responses by patients may decrease the therapeutic efficacy of a polypeptide — or worse. Neutralizing antibodies to interferon beta (used to treat multiple sclerosis), for example, led to higher relapse rates and more disease activity as measured by brain magnetic resonance imaging (MRI) scans (3, 4, 22, 23). Antibodies developed against recombinant erythropoietin (EPO) were shown to produce a potentially fatal side effect, the life-threatening condition known as “pure red-cell aplasia” in some patients (8).
In another study, 15–30% of hemophilic patients treated with recombinant human factor VIII (rFVIII) developed inhibitory antibodies toward that essential clotting factor. With hemophilia A, such neutralizing antibodies can cause life-threatening bleeding episodes and significant morbidity, necessitating treatment with a prolonged course of a tolerance-inducing therapy to reverse immunity (5,6,7). Other biotherapeutics shown to elicit unwanted immune responses include thrombopoietin (24); granulocyte macrophage–colony-stimulating factor (GM-CSF) (25); interleukin 17 (IL-17) (26); the monoclonal antibody (MAb) therapeutics infliximab (27), rituximab (28), adalimumab (29), and natalizumab (30); and other MAbs specific to tumor-necrosis factor (TNF) (31).
MAbs Pose a Special Problem: Although many biotherapeutic proteins exhibit a tendency toward aggregation, MAbs are especially subject to significant aggregation. This may be because their natural biological activity depends on strong protein–protein association. Relatively high therapeutic doses needed for clinical efficacy (1–3 mg/kg by weight) require substantially concentrated for
mulations (which exacerbate aggregation) to allow for intravenous administration of volumes that are small enough for patient convenience and comfort.
MAbs are highly effective therapeutics across a broad range of diseases in oncology, autoimmune, and inflammatory diseases, such that they now represent one of the largest and most rapidly growing segments of the pharmaceutical industry. At present, more than 30 therapeutic MAbs are marketed in the United States and Europe for a number of indications. US sales alone in 2010 reached ~$18.5 billion, and growth is expected to continue at a combined annual growth rate (CAGR) >10% (32, 33).
Drug makers are striving to reduce administration volumes further to minimize costs and increase patient compliance by making subcutaneous delivery possible. So it has become desirable to formulate MAb therapeutics at ever-increasing concentrations, which further necessitates inclusion of surfactants to prevent aggregation. It is estimated that ~70% of all MAb therapeutics currently include polysorbate 80 (34). The rate at which oxidative damage to proteins occurs is a function not only of the concentration of reactive oxidative species, but also concentration of the therapeutic protein itself. So the problem of oxidative damage is exacerbated by these newer, more concentrated formulations.
Polysorbates Pose a Unique Formulation Challenge: Polysorbates are not individual chemical species; they are mixtures of structurally related fatty-acid esters of polyoxyethylene sorbitan. Two principal fatty acids (lauric acid and oleic acid) compose up to 60% of the total fatty acid composition, with esters of other fatty acids and chain lengths making up the remainder of these molecules (34). Commercial polysorbate preparations contain measurable amounts of polyoxyethylene, polyoxyethylene sorbitan, and isosorbide polyoxyethylene fatty-acid esters, including polyoxyethylene sorbitan monooleate-dioleate-trioleate-tetraoleate, and polyoxyethylene isosorbide monoester-diester (35,36,37,38,39). The polyoxyethylene moieties and unsaturated alkyl chains in Tween polysorbate 80 (Figure 1) are sites of spontaneous autooxidation. Containing only saturated alkyl chains, Tween polysorbate 20 is less prone to generating certain oxidative species, but peroxide contaminants are easily demonstrated in Tween 20 samples.
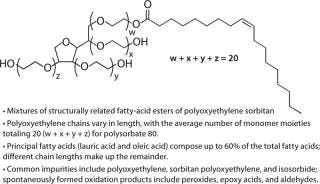
Figure 1: ()
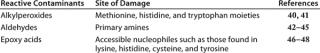
Table 2 lists oxidative species found in polysorbates along with the aminoacyl side chains that are affected by different reactive species. There is a great deal of variation in the nature and content of oxidative species found in different polysorbate lots. For example, one analysis of 14 lots of polysorbate 80 from four different manufacturers showed a 26-fold range in hydroperoxide content (290–7,700 nmole/g, with an average of 1,807 nmole/g) (23). The level of reactive contaminants in these preparations varies over time because autoxidation of polysorbates is spontaneous and progressive.
“High-purity” polysorbates are available commercially and marketed as such. Such preparations are treated to remove peroxides and packaged with oxygen excluded from their container headspace, where it is replaced with nitrogen or argon. Unfortunately, oxidation resumes as soon as the contents come into contact with oxygen, and that oxidation reaction accelerates once the polysorbates go into an aqueous solution. Typically, reactive species are detectable within one or two weeks following purification. Excluding oxygen from the manufacturing process and final packaging should in theory reduce oxidative damage overall (49), so this approach has been used for some biotherapeutics.
The amounts of polysorbate present in biotherapeutics are significant. Table 1 lists the molar ratios of polysorbate/API for some MAb examples and other biotherapeutics. Those ratios are substantial in some instances. For the five antibody products listed, the ratio ranges from a low of 0.46 for Herceptin trastuzumab to a high of 7.46 for Avastin bevacizumab. For the nonantibody biotherapeutics, the ratios range from 0.18 to 56.00. Each PS-80 molecule contains an average of 20 polyoxyethylene moieties. So depending on the extent of oxidative degradation, each such molecule could contribute more than one molar equivalent of reactive peroxides.
It is not meaningful to measure residual reactive contaminants in final biotherapeutic products because reactive species are used up in reactions with proteins. They form neoantigens within protein structures, creating nonsimilarity to self-molecules. If possible, it would be better to assess the degree of protein damage by looking for modified aminoacyl groups in a biotherapeutic over time during stability studies, which may ultimately become a regulatory requirement.
It is worth noting that other classes of surfactants useful in drug formulations — such as the poloxamers (typically known by trade names including Pluronics and Kolliphor from BASF and Synperonics from Sigma-Aldrich), Brij 35 detergent, and related molecules — also contain polyoxyethylene moieties, which are the source of spontaneously formed peroxides. Because those surfactants are less frequently found in biotherapeutics currently on the market, little work has been reported in the literature yet relating to their effects.
Methods for Determining Immunogenicity
Immunogenicity assessment is complicated and multidimensional for biotherapeutics. In recent years, a number of articles have been published dealing with the underlying philosophical approach to making meaningful determinations (50,51,52,53,54,55). Wadhwa and Thorpe provide an excellent summary, detailed description, and analysis of the pros and cons of the most well-accepted screening assays for antibody detection (55). The methods they included are direct enzyme-linked immunosorbent assays (ELISAs), bridging ELISAs, electrochemiluminescence assays, radioimmunoprecipitation assays, and surface plasmon resonance.
An underlying conclusion to be drawn from all of those studies is that no one method is adequate alone, so a combination of two or more such methodologies should be incorporated in a multitiered detection ap
proach. A growing number of reports concern additional methods of detecting and assessing antibody response involving the use gene expression and cell-based assays (56,57,58,59,60).
A Biosimilar Solution
Unwanted immunogenicity is a preeminent safety challenge associated with all biological therapeutics, and it can lead to unexpected and sometimes severe adverse effects. Polysorbate surfactants commonly used to prevent protein aggregation (a principal cause of unwanted immunogenicity) can contribute further to unwanted immunogenicity with chemically reactive species that are essentially ubiquitous contaminants of all commercially available preparations. Resulting chemical modification of aminoacyl side chains by those reactive contaminants leads to creation of neoantigens within protein structures. Because of their nonsimilarity to self-molecules, those can induce an immune response by activating antibody-secreting B cells and subsequently generate antibodies against a therapeutic. MAbs are a biotherapeutic class that is particularly prone to the aggregation problem because of the need to be administered at high doses in relatively small administration volumes.
Perhaps the two greatest technical challenges for biosimilar manufacturers will be demonstrating equivalent (or ideally superior) immunogenicity compared with their reference innovator products. Biosimilar makers must demonstrate that their excipient impurities are similar to those in the innovator products — or better yet, absent. Replacing polysorbates with non-autooxidizing surfactants can eliminate related lot variation in excipient composition and purity. That may limit resulting variations in immunogenicity caused by lot-to-lot differences in levels of chemically reactive contaminants. Certain alkylsaccharides highly effective in preventing protein aggregation have been put forth as nonoxidizing alternatives to polysorbates (61, 62) and are currently in development.
By eliminating polysorbates as a source of variable and progressively increasing immunogenicity, clinical-trial and postapproval lots are more likely to exhibit similar biochemical and immunogenicity characteristics. That should reduce the potential for product recalls later on. And this would seem to be a useful and fundamental element of any risk-management plan for new biotherapeutics — especially biosimilars and “biobetter” products.
About the Author
Author Details
Edward T. Maggio, PhD, is CEO of Aegis Therapeutics LLC, 16870 W. Bernardo Drive, Suite 390, San Diego, CA USA; 1-858-618-1400, x101; [email protected].
REFERENCES
1.) CDER/CBER.
2.) EMEA/CHMP/BMWP/114720/2009.
3.) Kappos, L. 2005. Neutralizing Antibodies and Efficacy of Interferon β-1a: A 4-Year Controlled Study. Neurol. 65:40-47.
4.) Vartanian, T, PS Sorensen, and G. Rice. 2004. Impact of Neutralizing Antibodies on the Clinical Efficacy of Interferon Beta in Multiple Sclerosis. J. Neurol. 251:1125-1130.
5.) Purohit, VS, CR Middaugh, and SV. Balasubramanian. 2006. Influence of Aggregation on Immunogenicity of Recombinant Human Factor VIII in Hemophilia A Mice. J. Pharm. Sci. 95:358-371.
6.) Hooks, WK. 2006. Urgent Inhibitor Issues: Targets for Expanded Research. Haemophilia 12:107-113.
7.) Reipert, BM. 2007. Mechanisms of Action of Immune Tolerance Induction Against Factor VIII in Patients with Congenital Haemophilia A and Factor VIII Inhibitors. Br. J. Haemophilia 136:12-25.
8.) Casadevall, N. 2002. Pure Red-Cell Aplasia and Antierythropoietin Antibodies in Patients Treated with Recombinant Erythropoietin. New Engl. J. Med. 346:469-475.
9.) Akers, MJ DeFilippis Hovgaard,. 2013.Peptide and Proteins As Parenteral SolutionsPharmaceutical Formulation Development of Peptides and ProteinsSecond Edition, CRC Press, Boca Raton.
10.) Donbrow, M, E Azaz, and A. Pillersdorf. 1978. Autoxidation of Polysorbates. J. Pharm. Sci. 67:1676-1681.
11.) Donbrow, M, R Hamburger, and E. Azaz. 1975. Surface Tension and Cloud Point Changes of Polyoxyethylenic Nonionic Surfactants During Autoxidation. J. Pharm. Pharmacol. 27:160-166.
12.) Donbrow, M. 1978. Development of Acidity in Nonionic Surfactants: Formic and Acetic Acid. Analyst (Lond.) 103:400-402.
13.) Ha, E, W Wang, and YJ. Wang. 2002. Peroxide Formation in Polysorbate 80 and Protein Stability. J. Pharm. Sci. 91:2252-2264.
14.) Wasylaschuk, WR. 2007. Evaluation of Hydroperoxides in Common Pharmaceutical Excipients. J. Pharm. Sci. 96:106-116.
15.) Liu, JY, YZ Li, and WB. Chang. 1999. Measurement of the Peroxidation of Brji-35 in Aqueous Solution By Hemin and Horseradish Peroxidase Catalyzed Fluorogenic Reaction. Fresenius J. of Analytical Chem. 365:448-451.
16.) Maggio, ET. 2012. Polysorbates, Peroxides, Protein Aggregation and Immunogenicity: A Growing Concern. J. Excip. Food Chem. 3:45-53.
17.) Ding, S. 1993. Quantitation of Hydroperoxides in the Aqueous Solutions of Non-Ionic Surfactants Using Polysorbate 80 As the Model Surfactant. J. Pharm. Biomed. Anal. 11:95-101.
18.) Nassar, MN. 2004. Influence of Formaldehyde Impurity in Polysorbate 80 and PEG-300 on the Stability of a Parenteral Formulation of BMS-204352: Identification and Control of the Degradation Product. Pharm. Dev. Technol. 9:189-195.
19.) Kishore, RS. 2011. The Degradation of Polysorbates 20 and 80 and Its Potential Impact on the Stability of Biotherapeutics. Pharm. Res. 28:1194-1210.
20.) Hvattum, E. 2012. Characterization of Polysorbate 80 with Liquid Chromatography Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy: Specific Determination of Oxidation Products of Thermally Oxidized Polysorbate 80. J. Pharm. Biomed. Anal. 62:7-16.
21.) Jefferis, R. 2011. Aggregation, Immune Complexes and Immunogenicity. MAbs 3:503-504.
22.) Giovannoni, G, FE Munschauer, and F. Deisenhammer. 2002. Neutralising Antibodies to Interferon B During the Treatment of Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 73:465-469.
23.) Bertolotto, A. 2004. Neutralizing Antibodies to Interferon B: Implications for the Management of Multiple Sclerosis. Curr. Opin. Neurol. 17:241-246.
24.) Li, J. 2001. Thrombocytopenia Caused By the Development of Antibodies to Thrombopoietin. Blood 98:3241-3248.
25.) Wadhwa, M. 1996. Production of Neutralizing GM-CSF Antibodies in Carcinoma Patients Following GM-CSF Combination Therapy. Clin. Exp. Immunol. 104:351-358.
26.) Cludts, I. 2010. Detection of Neutralizing Interleukin-17 Antibodies in Autoimmune Polyendocrinopathy Syndrome-1 (APS-1) Patients Using a Novel Non-Cell Based Electrochemiluminescence Assay. Cytokine 50:129-137.
27.) Baert, F. 2003. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s disease. N. Engl. J. Med. 348:601-608.
28.) Schmidt, E. 2009. Immunogenicity of Rituximab in Patients with Severe Pemphigus. Clin. Immunol. 132:334-341.
29.) Bartelds, GM. 2007. Clinical Response to Adalimumab: Relationship to Anti-Adalimumab Antibodies and Serum Adalimumab Concentrations in Rheumatoid Arthritis. Ann. Rheum. Dis. 66:921-926.
30.) Subramanyam, M. van der Weert, M 2001.Case Study: Immunogenicity of Natalizumab. Biotechnology: Pharmaceutical Aspects Immunogenicity of Biopharmaceuticals (Volume 8)Antibodies to Thrombopoietin. Blood:3241-3248.
31.) Aarden, L, SR Ruuls, and G. Wolbink. 2008. Immunogenicity of Anti-Tumor Necrosis Factor Antibodies: Toward Improved Methods of Anti-Antibody Measurement. Curr. Opin. Immunol. 20:431-435.
32.) Buss, NA. 2012. Monoclonal Antibody Therapeutics: History and Future. Curr. Opin. Pharmacol. 12:615-622.
33.).
34.) Hawe, A, V Filipe, and W. Jiskoot. 2010. Fluorescent Molecular Rotors as Dyes to Characterize Polysorbate-Containing IgG Formulations. Pharm. Res. 27:314-326.
35.) Kerwin, BA. 2008. Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways. J. Pharm. Sci. 97:2924-2935.
36.) Ayorinde, FO. 2000. Analysis of Some Commercial Polysorbate Formulations Using Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 14:2116-2124.
37.) Brandner, JD. 1998. The Composition of NF-Defined Emulsifiers: Sorbitan Monolaurate, Monopalmitate, Monostearate, Monooleate, Polysorbate 20, Polysorbate 40, Polysorbate 60, and Polysorbate 80. Drug. Dev. Ind. Pharm. 24:1049-1054.
38.) Frison-Norrie, S, and P. Sporns. 2001. Investigating the Molecular Heterogeneity of Polysorbate Emulsifiers By MALDI-TOF MS. J. Agric. Food Chem. 49:3335-3340.
39.) Zhang, R. 2012. Analysis of Polysorbate 80 and Its Related Compounds By RP-HPLC with ELSD and MS Detection. J. Chromatog. Sci. 50:598-607.
40.) Ji, JA. 2009. Methionine, Tryptophan, and Histidine Oxidation in a Model Protein, PTH: Mechanisms and Stabilization. J. Pharm. Sci. 98:4485-4500.
41.) Simat, TJ, and H. Steinhart. 1998. Oxidation of Free Tryptophan and Tryptophan Residues in Peptides and Proteins. J. Agric. Food. Chem. 46:490-498.
42.) Thliele, GM. 1998. Soluble Proteins Modified with Acetaldehyde and Malondialdehyde Are Immunogenic in the Absence of Adjuvant. Alcohol Clin. Exp. Res. 22:1731-1739.
43.) Moghaddam, AE. 2011. Reactive Carbonyls Are a Major Th2-Inducing Damage-Associated Molecular Pattern Generated By Oxidative Stress. J. Immunol. 187:1626-1633.
44.) Allison, ME, and DT. Fearon. 2000. Enhanced Immunogenicity of Aldehyde-Bearing Antigens: A Possible Link Between Innate and Adaptive Immunity. Eur. J. Immunol. 30:2881-2887.
45.) Lagergård, T. 2007. Formaldehyde Treatment Increases the Immunogenicity and Decreases the Toxicity of Haemophilus ducreyi Cytolethal Distending Toxin. Vaccine 25:3606-3614.
46.) Mateo, C. 2007. Advances in the Design of New Epoxy Supports for Enzyme Immobilization-Stabilization. Biochem. Soc. Trans. 35:1593-1601.
47.) Grazú, V. 2003. Novel Bifunctional Epoxy/Thiol-Reactive Support to Immobilize Thiol Containing Proteins By the Epoxy Chemistry. Biomacromolecules 4:1495-1501.
48.) Chu, JW. 2004. Understanding Oxidative Instability of Protein Pharmaceuticals. Mol. Eng. Biol. Chem. Systems (MEBCS) http://hdl.handle.net/1721.1/3955.
49.) Harwood, RJ, JB Portnoff, and EW. Sunbery Avis, KE, HA and L. 1993.The Processing of Small Volume Parenterals and Related Sterile ProductsPharmaceutical Dosage Forms: Parenteral MedicationsSecond Edition, Marcel Dekker, New York:70-73.
50.) Wadhwa, M. 2003. Strategies for Detection, Measurement and Characterization of Unwanted Antibodies Induced By Therapeutic Biologicals. J. Immunol. Meth. 278:1-17.
51.) Wadhwa, M, and R. Thorpe. 2006. Strategies and Assays for the Assessment of Unwanted Immunogenicity. J. Immunotoxicol. 3:115-121.
52.) Rosenberg, AS, and A. Worobec. 2004. A Risk-Based Approach to Immunogenicity Concerns of Therapeutic Protein Products: Part 1 — Considering Consequences of the Immune Response to a Protein. BioPharm Int. 17:22-26.
53.) Wadhwa, M, and R. Thorpe. 2009. The Challenges of Immunogenicity in Developing Biosimilar Products. IDrugs 12:440-444.
54.) Stas, P, and I. Lasters. 2009. Strategies for Preclinical Immunogenicity Assessment of Protein Therapeutics. IDrugs 12:169-173.
55.) Wadhwa, M, and R. Thorpe. 2012. Unwanted Immunogenicity: Lessons Learned and Future Challenges. Bioanalysis 2:1073-1084.
56.) Wei, X, SJ Swanson, and S. Gupta. 2004. Development and Validation of a Cell-Based Bioassay for the Detection of Neutralizing Antibodies Against Recombinant Human Erythropoietin in Clinical Studies. J. Immunol. Meth. 293:115-126.
57.) Bertolotto, A. 2007. Development and Validation of a Real Time PCR–Based Bioassay for Quantification of Neutralizing Antibodies Against Human Interferon-B. J. Immunol. Meth. 321:19-31.
58.) Moore, M. 2009. Measurement of Neutralising Antibodies to Type I Interferons By Gene Expression Assays Specific for Type 1 Interferon-Inducible 6–16 mRNA. J. Pharm. Biomed. Anal. 49:534-539.
59.) Lallemand, CJ. 2008. Quantification of Neutralizing Antibodies to Human Type I Interferons Using Division-Arrested Frozen Cells Carrying an Interferon-Regulated Reporter-Gene. J. Interferon Cytokine Res. 28:393-404.
60.) Kropshofer, H., and T. Singer. 2006. Overview of Cell-Based Tools for Pre-Clinical Assessment of Immunogenicity of Biotherapeutics. J. Immunotoxicol. 3:131-136.
61.) Maggio, ET. 2008. Novel Excipients Prevent Aggregation in Manufacturing and Formulation of Protein and Peptide Therapeutics. BioProcess Int. 6:58-65.
62.) Maggio, ET. 2013. Alkylsaccharides: Circumventing Oxidative Damage to Biotherapeutics Caused By Polyocyethylene-Based Surfactants. Ther. Deliv. in press 4.
You May Also Like





