October 2023 Featured Report
Experience with manufacturing viral vectors used for gene therapeutics is essential to mitigating risks in manufacturing of clinical trial materials. Collaborating with an experienced contract development and manufacturing organization (CDMO) with can help product developers design seamless and scalable vector-production processes. As authors from IDT Biologika describe here, a proven manufacturing platform and high-throughput analytical technologies help to ensure patient safety with products that meet high quality standards. The authors highlight the utility of digital polymerase chain reaction (dPCR), anion-exchange high-performance liquid chromatography (AEX-HPLC), size-exclusion chromatography with multiangle light-scattering (SEC-MALS), and next-generation sequencing (NGS) in gene therapy development.
Fill out the form below to read the full article now.
Sponsored Content
Modern Sensor Technologies for Viral-Vector Manufacturing: Improving Ultraviolet Photometric Detection of Full and Empty Adenoassociated Virus CapsidsModern Sensor Technologies for Viral-Vector Manufacturing: Improving Ultraviolet Photometric Detection of Full and Empty Adenoassociated Virus Capsids
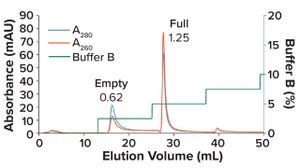
Despite rapid progress, significant obstacles remain for viral-vector manufacturing, including the need for effective separation of full and empty viral capsids. Optimizing that step is difficult but critical to achieving good yields of functional vectors. The biopharmaceutical industry has rapidly developed enhanced resins, matrices, and methods for such processes. As this author from EnVision Instruments explains, dual-channel, in-line photometers based on modern UV-LED light sources provide a powerful tool for process scientists working to address current limitations. He highlights the limitations of traditional mercury-vapor UV light sources — from unwieldy size to high power draw and the tendency to cause sensor drift — before describing the alternative that light-emitting diodes present. Narrow-bandwidth LEDs centered at or near the wavelengths of interest help analysts by overcoming the key limitation of legacy UV-filter photometers, and they are less resource intensive and operate in a smaller foo...
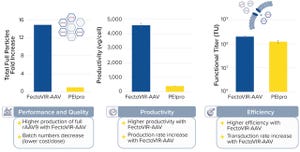
Although chemical synthesis enables production of modified DNA products at manufacturing scales that cannot be achieved using enzymatic technology, the latter offers benefits related to sustainability and flexibility. Enzymatic synthesis should be able to make longer sequences than can be made through using chemical methods. Biological synthesis has been used for many years to obtain micrograms to kilograms of nucleic acids (e.g., of double-stranded circular DNA plasmids). Methods based on phage systems can make linear and circular single-stranded DNA in bacterial fermentation. For applications requiring large amounts of unmodified DNA, that approach could be both efficient and cost-effective. As the chief scientific officer of genomics company Integrated DNA Technologies points out in this brief discussion, quality control is important no matter what method of production is used.
Recent successes in gene therapies are driving continued expansion of the pipeline. The number of clinical trials using adenoassociated virus (AAV) and lentivirus (LV) vectors is growing each year. Currently underway are 669 AAV and 266 LV trials in the United States, 1,250 AAV and 94 LV trials in Europe, and 223 AAV and 140 LV trials in the Asia–Pacific region (
1
). And as target indications broaden, many candidates treat conditions that affect much larger patient populations than before, calling for systemic delivery with larger doses. These advances are increasing the demand for AAV and LV vector manufacturing.
One major challenge is that current viral-vector manufacturing processes suffer from poor yields — typically 30–40%. Fortunately, opportunities exist to improve the performance of both upstream and downstream operations. A great deal of attention has focused on improving purification strategies, but more investigation can and should be put into upstream production. Improvements in such contexts...