WWW.LIFETOUCH.COM
This issue has been created by editors living in over-the-top levels of wildfire smoke here in Oregon’s Willamette Valley. Although all of us have been safe, we are appalled at the widespread loss of property and lives over these past two weeks. Amid those losses, and against the backdrop of political and social upheavals, the coronavirus continues to wreak its own havoc on just about every aspect of our lives. Adding natural disasters and subsequent loss of homes and possessions seems like the unkindest cut of all for so many people who already have lost employment and health benefits because of the pandemic.
For the past several months, we have prioritized content relevant to the international biopharmaceutical industry’s focus on coronavirus mitigation. We know that effective preventative vaccines as well as antibodies and/or other treatments for COVID-19 infections are prerequisite to the return to some kind of normal routine around the world. For many of us, we need those medicines ...
The Australian Research Council (ARC) Training Centre for Biopharmaceutical Innovation (CBI) at the University of Queensland in Brisbane, Australia
Global pharmaceutical industry research and development (R&D) investment has experienced steady growth over the past two decades, with an anticipated compound annual growth rate (CAGR) of 3.0% and projected 2024 investment of US$213 billion (
1
). Focused on developing innovative therapies for chronic, infectious, genetic, and lifestyle-related ailments, the fast-growing biologics segment has become a cornerstone of the pharmaceutical industry and healthcare sector. The demonstrated effectiveness and wide-ranging applicability of biopharmaceuticals also have brought considerable R&D in computational and biological technologies. Although such critical investments are contributing to an increased number of drugs in clinical trials, companies also are realizing the need to invest in other areas such as the future workforce.
Historically, the worlds of academia an...
Example of an alternating tangential-flow (ATF) technology as developed for perfusion culture systems by Refine Technology, now Repligen
(https://www.repligen.com/
technologies/xcell-atf)
Future biomanufacturing must address industry drivers, including the need for decreasing cost of goods (CoG), increasing market globalization, shortening development time for pipeline products, reducing risk to patient supply, and improving product quality. A CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies” took place on 16–17 July 2018 in Gaithersburg, MD, to address those opportunities.
Advanced technologies include single-use bioreactors, alternating tangential-flow (ATF) systems used during fermentation, modular and closed process equipment, process analytical technologies (PAT), and other analytical systems. When used in future biomanufacturing, such systems should enable robust processing, reduce waste, incre...
Figure 1: Overview of timeline from proof of concept to single-use prototype
The production of increasingly higher cell densities has stressed the already limited solids-handling capabilities for traditional intermittent ejection centrifuge systems. By contrast, a single-use disc-stack centrifuge based on the solids-flow principle offers distinct advantages for cell culture harvesting. Such benefits include solids handling of high-density cell culture processes and elimination of the separation disruption and aerosol generation associated with the intermittent solids ejection. A single-use system also provides well-established benefits of disposable components — such as removal of steam- and clean-in-place (SIP/CIP) operations — while enabling a fully closed centrifuge system through the elimination of a discharge mechanism.
Figure 2: Theoretical yields as function of feed packed cell volume (PCV) and heavy-phase (HP) PCV
Design Approach
To succeed with a fully contained continuous solids-transportation s...
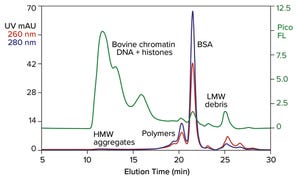
Figure 1: Analytical size-exclusion chromatography (SEC) of bovine serum albumin (BSA) marketed for processing nucleic acids; sample prestained with PicoGreen dye; TSKgel G4000SWxl, 7.8 mm × 30 cm, 50 mM MES, 150 mM NaCl, 0.05% Pluronic reagent F68, pH 6.5; flow rate: 0.5 mL/min.
A high-performing capture method is a critical bedrock asset for developing industrial purification processes. This is especially true for extended families of products that share highly similar chemical composition. Therapeutic monoclonal IgG is an example. The ability of protein A affinity chromatography to achieve 95% purity in one simple step was the runway that got recombinant immunotherapy off the ground and made it available to millions.
In fact, protein A did more. Beyond giving the industry a foundation manufacturing method, it made IgG purification accessible to immunologists without the need for advanced purification skills. It allowed them to screen efficacy, stability, pharmacokinetics, and various aspects of manufac...
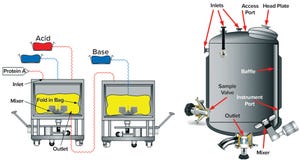
Figure 1: Examples of equipment used for low-pH viral inactivation; (top) a single-use bag system with an integrated mixer; (bottom) a typical stainless-steel tank displaying common appurtenances such as inlets, outlets, thermowells, and so on (
photos from Sartorius and Feldmeier
)
Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such operations, it is unsurprising to see a range of segregation strategies adopted by different companies.
Herein we ...
Dye-Stripping Buffer and Resin Stripped-Dye Analysis: Development and Optimization of a Novel Spectrophotometric Assay and Method for Removal of Cibacron Blue DyeDye-Stripping Buffer and Resin Stripped-Dye Analysis: Development and Optimization of a Novel Spectrophotometric Assay and Method for Removal of Cibacron Blue Dye
Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (
1
). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient, compromising product quality and patient safety.
Chromatographic purification of therapeutic proteins often begins with affinity chromatography to achieve high purity and recovery in a first step. Affinity binds only the protein of interest and thus removes many impurities including proteases. Often the affinity interaction incorporates a low-pH viral inactivation unit operation in the same...
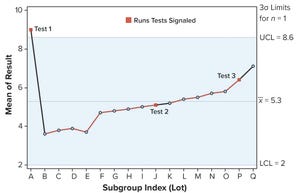
Figure 1: Individuals (X) chart of results; subgroup sizes
n
= 1; UCL = upper control limit, LCL = lower control limit
Control charts can be used to assist in monitoring of biopharmaceutical product quality attributes as part of continued process verification activities. A number of tests known as run rules have been developed to assess whether biomanufacturing processes remain in statistical control. In practice, results for such attributes can be positively autocorrelated. Simulated data are used to assess the performance of run rules with autocorrelated data to assist in determining risk–reward profiles for process monitoring.
Autocorrelated Data
The tendency for data to be positively autocorrelated (values are closely related to each other in sequential order) in biopharmaceutical manufacturing processes and the effect of such data on the performance of run rules have been highlighted in two 2019 studies (
1, 2
). The simulation strategy used herein is based on a Monte Carlo approach to represent ho...
Quality risk management (QRM) is a systematic process for assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. Although QRM is not new (
1
), the regulatory focus on QRM will increase with the arrival of the European Medicines Agency’s (EMA’s) Annex 1 (
2
), which was reviewed by the US Food and Drug Administration (FDA), the World Health Organization (WHO), and the Pharmaceutical Inspection Convention Scheme (PIC/S). Integrity testing of sterilizing-grade filters is a key focus of QRM because it is a fundamental element of sterility assurance.
The following discussion points out the insufficiency of traditional QRM for filter-integrity testing and advocates for a comprehensive approach. Such a method offers thorough identification of possible failure modes, ways to prevent failure, and improved detectability through use of program-specific parameters for automatic detection of abnormal conditions.
Why QRM?
More than just a regulatory req...
Adenoassociated virus (AAV) vectors are a popular choice for modern gene therapies because of their favorable safety profile, low immunogenicity, and the ease with which they can be transduced into different cell and tissue types. An AAV genome is a single strand of DNA comprising a replication (rep) gene, which encodes regulatory proteins involved in genome replication, and a capsid (cap) gene, which produces three capsid proteins.
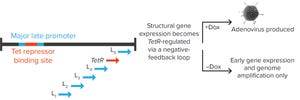
Figure 1: Tet repressor (TetR) binding site is inserted into the major late promoter, and the TetR gene itself is encoded within the late region. That creates a negative feedback loop, in which adenoviral structural proteins can be produced only in the presence of doxycycline (Dox). Without it, TetR binding inhibits expression of adenoviral late-region (structural) genes.
However, AAVs cannot replicate alone. In nature, AAV shares an exquisite relationship with adenovirus, which provides most of the functions needed for the AAV lifecycle. That highly evolved and dynamic relati...
Flow-through anion-exchange (AEX) chromatography is used frequently in biopharmaceutical purification processes for reduction of net–negatively charged host-cell proteins (HCPs) and viruses as part of a validated viral clearance strategy (
1, 2
). AEX column chromatography is the technology most often used for electrostatic viral clearance, particularly in commercial-scale biopharmaceutical manufacturing, for which columns have a long-established history of reliable and well-understood performance (
3
). Still, validation of HCP and viral clearance by AEX columns in biopharmaceutical processes involves complexities that contribute significantly to operational and regulatory costs. Chromatography columns generally are used over tens to hundreds of purification cycles, with intervening cleaning procedures that can degrade resins. Biomanufacturers must be concerned about the potential for loss of HCP capacity or viral clearance due to fouling or degradation with reuse (
4–7
). An assessment of the effect of ...
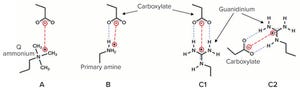
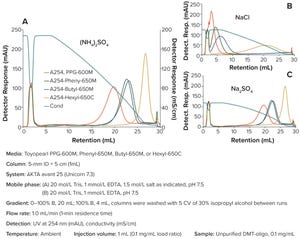
Within the biopharmaceutical industry, oligonucleotide drug pipelines have increased significantly because of the effectiveness of such drug products in treating devastating diseases. Downstream specialists need improved purification techniques for such highly valuable materials.
Figure 1: Impact of different salt conditions on hydrophobic-interaction chromatography (HIC) oligonucleotide purification
Dimethoxytrityl (DMT) is used to synthesize oligonucleotides and temporarily mask the characteristic chemistry of the 5ʹ-hydroxy functional group. DMT can be left on an oligonucleotide following synthesis to provide stability to a molecule during subsequent processing.
Herein, we describe a novel, effective, and high-recovery method for purifying a DMT-on oligonucleotide and effectively removing a DMT group from an oligonucleotide in a single purification step. This purification can be achieved by using hydrophobic- interaction chromatography (HIC) because the DMT-on group is strongly hydrophobic.
Materials a...
Qualitative and quantitative analyses of process-related impurities are critical to manufacturing a safe, high-quality drug product. During his 17 June 2020 “Ask the Expert” webcast,
Steven Broome
(BioPharmaSpec) described using mass spectrometry (MS) for such analyses. Focusing on isopropyl-β-D-thiogalactopyranoside (IPTG), kanamycin, and host-cell proteins (HCPs), Broome overviewed best practices for using MS to detect and quantitate low levels of impurities.
Broome’s Presentation
Chemicals used in biotherapeutic production can be present as impurities in the resulting drug product. These impurities can include inducers, antibiotics, chemical-reaction products, HCPs, and host-cell DNA. The presence of process-related impurities can raise immunogenicity concerns and diminish product potency, efficacy, and longevity. Thus, sponsors should identify impurities and estimate their concentrations early in drug development. Known impurities can be monitored during processing to demonstrate clearance and optim...
Biotherapeutics are a hot topic right now — and for good reason. But even before the COVID-19 pandemic, the biotherapeutics field, like every other manufacturing sector, was exploding with all the data that are being generated by recent innovations in equipment, systems, and processes. Advances in biomanufacturing analytics, analytical technology, and machine learning have tried to keep pace; however, such tools too often are misunderstood and applied suboptimally. Thus, many companies struggle with confusion and missed opportunities when they should be reaping the benefits of improved workflows and reliable product manufacturing.
Done right, applications of data analytics and machine learning can provide bioprocessing predictions and classifications — e.g., for assessing whether product quality meets specifications. If an analysis reveals that something is awry, the cause can be identified, whether it is in processes or raw materials. Data analytics and machine learning techniques also can bolster other ...