November-December 2019
The BWB exhibit hall; image credit: Scott Chernis and Ross Stacey
Over a decade ago, the BioProcess International Conference and Exhibition was created by combining formerly separate IBC conferences on upstream, downstream, analytical, and manufacturing topics for the biopharmaceutical industry. They became tracks in the larger program that reflect the coverage of the magazine after which the event was named. Over time, US West, Asian, and European variations on the theme were added to our yearly calendar. And in the past few years, Biotech Week Boston has evolved by similarly bringing together related meetings and events into a week-long fall festival of biotechnology. This year, BPI US East was colocated with a Cell and Gene Therapy Bioprocessing and Commercialization conference, artificial intelligence and digital health meetings, Xcelerate programs and the Xconomy awards, the Biopharm America partnering event, a Microbiome Therapeutics conference, a rare-disease film festival, the fifth annual Complia...
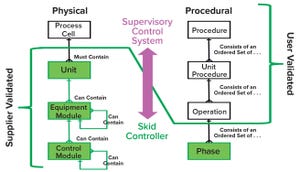
Figure 1: Physical and procedural model implementation based on principle 88 of International Society for Automation (ISA) standards for batch process control
Automation can improve efficiency, track performance, adjust operations, and liberate operators from mundane routines. Automation requires a flexible set of tools that align well with the inherent flexibility of single-use technology (SUT). Although SUT flexibility enhances a biomanufacturer’s ability to modify operations to meet the needs of today’s dynamic industry, it also increases timelines and costs related to customizing and validating automated additions.
We present herein the findings of a team of industry automation experts who are sharing their experiences within the BioPhorum Operations Group’s Technology Roadmapping collaboration to test new automation methods
(1)
. Our group’s vision is to move from the current state of unique and custom software development to a reusable, standardized approach that enables rapid integration of intell...
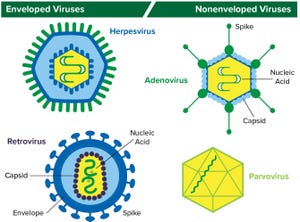
Figure 1: Structure of enveloped viruses (represented by herpesvirus and retrovirus) and nonenveloped viruses (represented by adenovirus and parvovirus) www.istockphoto.com
Viral contamination is a common threat to all animal- and human-derived biopharmaceuticals. This type of contamination can affect any part of a bioproduction process, so biomanufacturers need to perform viral testing studies and incorporate viral clearance methods into their processes.
Viral contaminants can come from cell lines (e.g., endogenous retroviruses) or from adventitious (e.g., mycoplasma) introduction during drug manufacturing. Virus testing of master cell banks (MCBs), working cell banks (WCBs), end-of-production cell banks, and bulk unprocessed harvest material is called for in guidance documents including Q5A from the International Council on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH)
(1)
. Regulators also recommend that manufacturers source raw materials appropria...
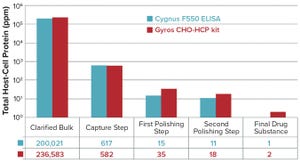
Figure 1: Comparing the Gyros Chinese hamster ovary host-cell protein (CHO HCP) kit with the CYGNUS F550 enzyme-linked immunosorbent assay (ELISA) kit for case 1
In the past few years, increasing numbers of biotherapeutics have been approved for market
(1)
. Among all the regulatory concerns for commercial biotherapeutics, host-cell proteins (HCPs) are a major class of process-related impurities that remains a critical quality attribute (CQA) for bioprocess development because of associated risks to product quality, safety, and efficacy. HCP identification, clearance, assay setup, and process control are critical points for health authorities, and many guidelines aim for better control of HCP content in final biologic products
(2–5)
. Through BioPhorum, the biotechnology industry has released a guideline and a risk assessment tool
(6, 7)
that covers management of HCPs during bioprocess development.
Several recently published studies have illustrated the diversity of HCPs and related issues with final ...
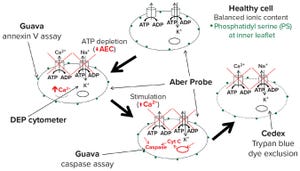
Figure 1: Evaluation of alternative cell counting methods; DEP = dielectrophoresis
Trypan blue dye exclusion first was proposed as a means of measuring mammalian cell damages over a century ago in 1917
(1)
. Despite extensive documentation of its limitations
(2)
, it remains the “gold standard” method of measuring cell viability in common use today. But can this method truly measure viability? And how do we define cell viability, for that matter? Those fundamental questions are linked to whether we refer to cells as “alive” or “dead” in the context of bioprocessing and how that relates to their ability to produce the recombinant proteins or viruses that we may want to use as biotherapeutics or vaccines.
“Happy” Cells
Researchers and conference presenters often refer to “happy” cells as those appearing to be normal under a microscope and that grow at an expected rate and secrete desired proteins. Of course those properties are associated with viable cells, and a textbook definition of viability would inc...
Susan Riley
is vice president and general manager of Advanced Bioprocessing. It’s been a year since Thermo Fisher Scientific’s acquisition of the Advanced Bioprocessing business from Becton Dickinson (BD).
Why did Thermo Fisher see the Advanced Bioprocessing (AB) business as a good fit with its life-science offerings?
AB has a significant portfolio in premium supplements for cell culture and microbial fermentation. The AB business was seen as a good fit for several reasons: It goes hand-in-glove with Gibco media, for which Thermo had used many AB supplements in those formulations. AB also offered analytical capabilities and a depth of material characterization and media analysis for understanding key drivers of cellular metabolism.
The AB business also brought manufacturing assets, including an animal-origin–free facility in Miami that had been the site of a large-capacity pharmaceutical company. That facility was appealing because it could help support future growth in the cell culture business. AB als...
Regulations require that biomanufacturers assess the intactness of protein and glycoprotein products as well as confirm the terminal sequences to look for existing variations. ICH Q6B guideline section 6.1.1 c states:
Terminal amino acid analysis is performed to identify the nature and homogeneity of the amino- and carboxy-terminal amino acids. If the desired product is found to be heterogeneous with respect to the terminal amino acids, the relative amounts of the variant forms should be determined using an appropriate analytical procedure. The sequence of these terminal amino acids should be compared with the terminal amino acid sequence deduced from the gene sequence of the desired product.
BioPharmaSpec’s protein characterization services include
Protein Terminal Structure
Proteins comprise linear chains of amino acids linked to one another through amide bonds. Each amino acid has an amine functional group at one end and a carboxylic acid functional group at the other. Linking the carboxylic acid group...
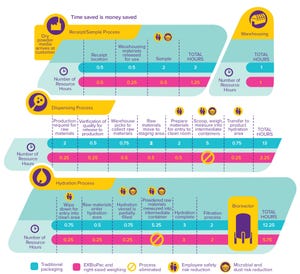
Figure 1: Media containers are available in four sizes up to 100 kg.
Although the handling and preparation of cell culture media can seem routine, a number of risks are associated with such operations. Identification and mitigation of associated risks can help ensure consistency of performance, minimize likelihood of contamination, and protect employees while enabling greater efficiencies in upstream processes. Here we describe a number of strategies for reducing risks and streamlining media-related workflows.
Simplifying Handling of Cell Culture Powders
Media preparation typically is quite labor intensive and poses risks related to containment and employee safety. In response to those challenges, we have engineered right-sized weighing to eliminate the need for our customers to weigh powder from bulk containers such as barrels and buckets. Our goal was to ensure delivery of high-quality cell culture media with precise weighing in an easy-to-use delivery system. We selected the EZ BioPac® transfer bag by ...
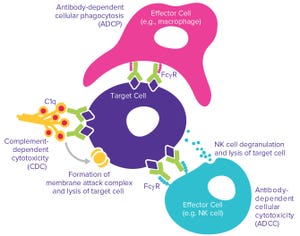
Figure 1: Monoclonal antibodies (MAbs) exert their biological effects through both Fab‑and Fc-mediated activities.
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics.
High-resolution analytical techniques are required to answer questions regarding product heterogeneity with respect to charge, size, glycan structure, and other posttranslational modifications. In addition to those analyses, biological characterization helps scientists answer critical questions about how a drug functions and provides insight into it...
Based on the many forms that modern vaccines can take, their manufacturing is complicated. Unlike monoclonal antibodies (MAbs), vaccine manufacturers have no “template” platform to follow. Most vaccine producers develop their manufacturing processes from scratch, a prospect that can be challenging for small to mid-sized companies.
Bioprocessing is the key challenge in vaccine manufacturing. Without a well-developed and understood process, a manufacturer will face serious challenges in commercial production: e.g., low yields, high costs, and difficulties in meeting quality standards.
Most vaccine-related training organized by universities focuses more on general product commercialization. Until recently, no available training addressed detailed technical challenges in vaccine bioprocessing —and the demand for such training is very high.
Take China for example: Most of its ~40 vaccine producers are small to mid-sized companies with limited resources for training. Many vaccines made in China use processes fr...
Viruses and virus-like particles (VLPs) raise unique challenges for downstream recovery and purification. In an Ask the Expert webinar on 19 September 2019,
Mark Snyder
(R&D applications manager of process chromatography at Bio-Rad Laboratories) discussed purification methods engineered to perform at manufacturing scales. Offering case studies featuring Nuvia resin and CHT Ceramic Hydroxyapatite chromatography support, Snyder emphasized that scalable media can help companies optimize downstream operations and address evolving manufacturing requirements.
Snyder’s Presentation
Tremendous growth in markets for vaccines and gene therapies is compelling manufacturers to generate more drug product and to work with greater numbers of viruses and VLPs than ever before. But perennial downstream challenges remain. At manufacturing scales, the size and structural complexity of such species can diminish efforts to recover and purify drug substance. Careful selection of chromatography resins can mitigate those chall...
Software risk management often seems synonymous with extra documentation. But during a 17 October 2019 Ask the Expert webinar,
Martin Laferriere
(director of information technology services at Avid Bioservices) explained how a thoughtful risk- assessment process at early project management stages can optimize software system validation efforts. Laferriere illustrated the benefits of developing strategic structures for documenting system use, hazards, and testing requirements. Such structures can help biomanufacturers clarify what records and functionalities they need to concentrate on to remain compliant. Then those companies can reduce their testing efforts while still robustly mitigating risk and remaining compliant.
Laferriere’s Presentation
Effective risk management demands careful evaluation, and software system compliance requires structured but flexible risk management to obtain useful and regulatory-compliant results. Thus, biopharmaceutical companies need to evaluate system capabilities consist...
The development and manufacture of emerging viral therapies is highly dependent upon accurate infective virus titers as well as total particle counts. But traditional quantification technologies have not kept pace with the needs of the viral vector market. Quantification methods based upon infectious particle counts can easily underestimate total particle counts. Indirect measurements such as enzyme-linked immunosorbent assays (ELISAs) and quantitative polymerase chain reaction (qPCR) are prone to overestimating the number of virus particles because they measure virus components to derive titer rather than enumerating full viruses and particles.
Antje Schickert
(virus analytics product manager at Sartorius Stedim North America, SSB) delivered an “Ask the Expert” webinar on 17 September 2019 to explain how her company’s Virus Counter 3100 platform enumerates virus particles more quickly, directly, and precisely than traditional methods.
Schickert’s Presentation
Precise virus quantification is difficult b...
Essential considerations for recruiting healthy donors for high-quality cell therapy manufacturing
The cell and gene therapy (CGT) segment has grown tremendously over the past decade. And while the industry deals with a steep learning curve inherent to a rapidly developing field, problems must be solved, and solutions must be reduced to practice. One such problem, long understood by the industry but now thrust into the spotlight, is sample handling: proper collection, processing, preservation, storage, and transportation of cellular starting material. Suboptimal techniques for such logistics have been shown not only to diminish cell viability and product efficacy, but also to introduce significant stumbling blocks to the process of getting new therapies approved
(1, 2)
.
Another crux is that the CGT industry increasingly requires high-quality starting material. Good drug products start with good raw materials, and in no case is that truer than for CGT. Human cells and tissues are the raw materials needed...