In this way-too-interesting time we’re living through, we are seeing the worst and best of humanity as we do what we can to protect ourselves and our families. When we can congregate again, some of us will be relieved to abandon home offices and get back to familiar work surroundings, social interactions, dependable Internet access, and company-provided toilet tissue. Others will have learned that working from home is the greatest thing next to sliced bread and lobby with their management to maintain what they have learned about new levels of productivity.
Although our parent company, Informa, sustains many publications (most of them Taylor & Francis academic journals), it is above all an events company. We often ask authors to expand on key elements of their conference presentations. So we are delighted with the transparent approach of our corporate officers toward communicating with employees and stockholders regarding the health and continuation of our group’s events. Postponing an event is a major und...
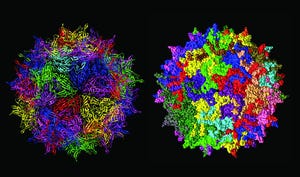
Structure of adenoassociated virus, often used as a gene therapy vector (adapted from https://commons.wikimedia.org)
Gene therapy
is defined as the transfer of genetic information to a patient for treatment of a disease. Clinical investigation of such therapies began in 1990 with a treatment for a rare immunodeficiency disorder and since has expanded to almost 1,000 clinical studies in 2019 (
1
,
2
). In its most straightforward incarnation, the goal of gene therapy for genetic diseases is long-term expression of a transferred gene at levels that are high enough to be therapeutic, an approach sometimes called
augmentation gene therapy
. Transferred genes are most frequently normal copies of a mutated gene. Treatments also can suppress expression of detrimental genes through RNA interference or genome-editing tools. New editing technologies are opening up the possibility for correcting mutated genes in their precise genomic locations through homologous recombination with a donor template or use of base-...
Anthony Davies of Dark Horse Consulting Group hosted the super plenary at Phacilitate 2020 (
www.phacilitate.co.uk
)
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics — and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week in Miami, FL), he reflected on difficulties the advanced-therapies sector has faced since its high of 2017, when three products achieved US Food and Drug Administration (FDA) approval: Kymriah (tisagenlecleucel) for Novartis, Yescarta (axicabtagene ciloleucel) for Kite Pharmaceuticals and Gilead Sciences, and gene therapy Luxturna (voretigene neparvovec...
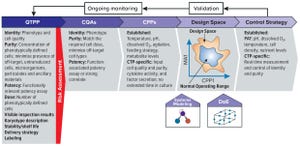
As advanced therapies, including regenerative medicines, progress toward commercialization and market approval, early warnings from key opinion leaders (
1
,
2
) regarding the importance of better understanding quality target product profiles (QTPPs) and critical quality attributes (CQAs) of such products have resounded ever louder (see the “Terminology” box for definitions). Costly late-stage delays, redirections, and even abandonment of clinical programs can be linked to quality issues associated with inadequate understanding of process and product. Therefore, a review of the benefits of a quality by design (QbD) approach to optimizing and informing the production processes is needed to reinforce the necessity of building quality into a process and to provide a framework in which early indications of quality issues can be ascertained (
3
).
The cell and gene therapy industry has experienced incredible growth and achieved significant milestones in recent years, including the rapid progress from early cl...
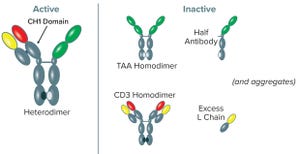
Figure 1: Variant forms of BsAb CD3-TAA–expressed products; the CD3-TAA heterodimer (containing one light (L) and two heavy (H) chains) is the active form. Other forms include the TAA homodimer, CD3 homodimer, half-antibody, and L chain, all of which are inactive. Aggregated forms of product also are present. Note that only the full-length anti-CD3 human heavy chain has a CH1 domain, and the anti-CD3 human heavy chain is present in only two forms: the CD3-TAA heterodimer and CD3 homodimer.
Bispecific antibodies (BsAbs) are designed to recognize and bind two different antigens, in many cases for the purpose of immune effector-cell activation to destroy cancer cells (
1
). Such BsAbs mediate cell killing by binding simultaneously to an antigen that is overexpressed on tumor cells and to the CD3 receptor, activating cytotoxic T lymphocytes (
2
). Using proprietary UniRat human heavy-chain technology combined with OmniFlic human fixed–light-chain antibody technology licensed from Ligand Pharmaceuticals, Teneo...
The dynamic binding capacity (DBC) of a chromatography resin represents the total amount of target protein that the resin will bind under actual flow conditions before significant breakthrough of unbound protein occurs. This is a useful parameter for predicting what the process performance of a resin will be in actual use.
DBC affects the overall amount of resin that can be packed in a given column for a process — and the number of batches that can be processed cost-effectively in manufacturing each year. If resin DBC is low, then more gel must get packed inside a column, ultimately increasing the cost factor involved in running a batch. For resins with higher DB values, less can be packed inside a column, thereby decreasing the overall utility cost of each batch run.
DBC can be determined using either partly purified monoclonal antibodies (MAbs) or cell culture feedstock. If the former are used, then the breakthrough curve can be measured directly by monitoring UV absorbance. Resin DBC also can be evalua...
Performing viral clearance studies is an important safety element of manufacturing all biopharmaceuticals expressed from mammalian cells (
1
). Typically, viral clearance is described as a log reduction value (LRV) and calculated as the log
10
of the ratio of input to output virus load. Amounts of virus load are calculated from the volume and concentration of input and output fractions.
Virus concentration is often calculated as 50% of tissue-culture infective dose (TCID
50
) using the Spearman–Kärber (SK) equation (
2
,
3
). In this method, replicate wells are inoculated with decreasing concentrations of test material, resulting in a transition from all wells being infected to no wells being infected. Based on the ratio of wells infected at different concentrations of test material, TCID
50
is calculated. If 10-fold dilutions are applied, and each well is inoculated with 0.1 mL, the SK equation becomes Equation 1 (next page), in which
ri
is the number of positive wells in row
i
, and
ni
is the to...
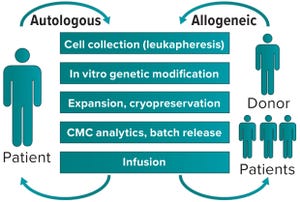
Figure 1: Autologous (left) and allogeneic (right) generation of cell therapy products
Initial progress in cell and gene therapy has seen 12 advanced therapeutic medicinal products (ATMPs) become available on the market in 2019 for a range of conditions, from monogenic diseases to cancer. Despite such progress, development of clinically and commercially successful cell therapies presents manufacturability challenges and questions about bypassing patients’ immune systems. The availability of rapid sequencing and next-generation bioinformatics has made it possible to understand the mechanisms of disease better and accelerate development of therapeutic responses. The same toolbox will enable our fight against cancer (with advanced immunotherapies) and other conditions.
The Autologous Approach
In cell therapy for immunooncology, often the medicinal product is a cell that has acquired a therapeutic function through genetic modification induced by a virus. That is achieved by collecting relevant cells from dono...
Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (
1
). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (
2
). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according to different agitation principles and gassing strategies (
3
). Therefore, each scale might require several runs to obtain similar results, and optimization also might be required at the different scales.
Scale-up challenges occur when bioreactors used during process transfer differ significantly in geometry and design. Different mixing methods, impeller types, and sparging strategies all can compromise ce...
As a full-service contract development and manufacturing organization (CDMO) specializing in gene therapy development, Yposkesi produces recombinant adenoassociated virus (AAV) and lentivirus (LV) vectors using adherent-based and suspension-adapted cell expression platforms. Alain Lamproye joined the company as chief executive officer (CEO) in January 2017, having served previously as president of the biopharmaceutical business unit of Novasep (2012–2017) and as CEO of Henogen, its subsidiary dedicated to gene therapy. He has held managerial positions in pharmaceutical operations at Merck Serono (including site director in Billerica, MA) and Eurogentec (as GMP production director). Lamproye holds an MS in biology from the University of Liège in Belgium.
AAV Vector Production
Scale-Up Issues:
Cost-effectiveness of large-scale manufacturing for viral vectors is a key challenge in gene therapy development. How are your development teams working to improve productivity of AAV vector production? And what can...
Cryopreservation provides critical protection for cell therapies by minimizing genetic changes. But cooling too slowly or quickly risks diminishing cell viability upon thaw. On 11 March 2020,
Peter Kilbride
(senior research scientist) and
Julie Meneghel
(cryobiologist), both of Cytiva (formerly GE Healthcare Life Sciences), discussed the importance of controlled-rate cryopreservation. Illustrating how mammalian cells change when frozen, Kilbride and Meneghel offered concrete cryopreservation strategies and identified temperatures at which it is safe to stop controlled cooling and transfer drug product to cryogenic storage.
The Presentation
Every cell system cools differently depending on membrane permeability, dehydration capacity, and tolerance for cryoprotectant. Because differences abound, even within cell types, it is critical to determine an optimal cooling rate before cryostorage. Freezing must proceed in a controlled, linear way to maximize postthaw viability.
Effective cooling balances drops i...
Sean Liour
(vice president for project management at GenScript ProBio) delivered an Ask the Expert presentation on 18 March 2020 to explore the advantages of his company’s platform for cell-line development. Liour explained that successful biologic development hinges on robust host cell lines, capable expression vectors, and discriminating clone-screening systems. Overviewing relevant technologies and capabilities, Liour illustrated how his company’s ProCLD platform helps sponsors navigate cell-line development.
Liour’s Presentation
Researchers must weigh their options carefully when selecting a host cell line because that decision will facilitate or hinder future operations, ultimately influencing cost of goods (CoG) and product quality. Hosts should be selected to facilitate process development and good manufacturing practice (GMP) production. For instance, high titers are essential, as is a short timeline from transfection to clone selection. But successful hosts perform consistently well over time: C...
Image: iStock/AlexLMX
Innovative drug manufacturers require an opportunity to recoup capital and opportunity costs that they incurred to develop new medicines. Once patents have expired, competitors should be empowered to promote widespread affordability. An exclusivity period followed by a competitive market would promote the otherwise incompatible objectives of incenting innovation and promoting affordability.
That careful balance exists for small-molecule medicines but not yet for biologics. The innovation side of the biologics market is working as intended, but a robust market for lower-cost competitors — biosimilars — has been slow to develop in the United States. Biosimilars are approved to compete in nine biologic classes but currently are available in only four. Biosimilars account for <1 % of total biologic sales volume (
1
). Thus, competitive pressures are not driving down costs sufficiently in the high-cost biologics market.
Methodology for Estimating Biosimilars Savings
In March 2020 (
2
), I...