WWW.LIFETOUCH.COM
My first business trip each year usually is to Washington, DC, in January. There, the CMC Strategy Forum and WCBP symposium (sponsored by CASSS have an excellent track record of setting the stage for topics that will play out through the rest the year. The combined event draws significant US Food and Drug Administration participation. So we also look forward to their help in focusing our attention on key regulatory issues and updates.
With continuing focus on well-characterized biologics, WCBP centers on assessments of comparability and stability, with emphasis on regulatory expectations. Products discussed this year included proteins, antibodies, vaccines, bi- and multispecifics, and cell/gene therapies. Analytical discussions highlighted ways to reduce time and costs of development, highlighting promising new technologies and reinforcing the benefits of collaborations of all types.
Regulatory sessions at WCBP have included more speakers each year from countries still developing their o...
www.istockphoto.com
The biopharmaceutical industry continues to grow at a rapid pace. That growth is accelerating even more because of keen investor and research interests in cell and gene therapies. My focus below is on several key growth areas in the field, specifically aspects of development and biomanufacturing. This discussion builds on my 2018 column on top trends in bioprocessing (
1
). In that article, I identified five key trends that continue to resonate now:
Figure 1: Investigational new drug (IND) applications in cell and gene therapy (CGT) to the US Food and Drug Administration (FDA) by year, 1990–2018; the Center for Biologics Evaluation and Research (CBER) had more than 800 active applications by October 2019.
Cell and Gene Therapy
The relatively new area of so-called “advanced therapies” has become the hottest ticket in biopharma town. Thanks to some impressive successes in both cancer and rare-disease therapies — and now with four approved products in the United States — companies are scr...
Figure 1:
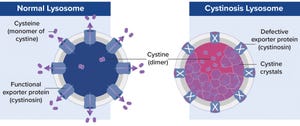
A defective gene encodes the exporter protein cystinosin, which causes cystine crystals to build up in lysosomes, thus damaging tissues and organs.
Lysosomal storage diseases are caused by mutated genes that express defective lysosomal proteins, such as essential enzymes. For example, cystinosis is a metabolic disease caused by a defective gene that encodes cystinosin, an exporter protein (Figure 1).
Avrobio develops gene therapies to treat lysosomal storage diseases. On 8 October 2019, the company announced that its first patient had been dosed in the AVR-RD-04 investigational gene therapy program, which involves genetic modification of the patient’s own hematopoietic stem cells to treat cystinosis. The company also has clinical-stage programs in Fabry disease and Gaucher disease, both of which are also lysosomal storage diseases. Avrobio’s technology platform includes an automated, closed-system process for manufacturing gene therapies. Below, Kim Warren (head of operations) discusses the use...
Scientists collaborating in R&D at adMare BioInnovations in Vancouver, BC
Canada’s life-science industry is exceptional at producing innovative research. With less than 0.5% of the world’s population, our country produces 5% of the globe’s total research publications. Canada’s citation rate ranks among the top six nations and is 43% higher than the global average (
1
). At the same time, our life-science industry is competing with other global jurisdictions into which investment is flowing. The US state of Massachusetts, already the number one biotechnology cluster in the world, has committed US$1.5 billion since 2008 to maintain its dominance. The San Francisco Bay area in California, now the second highest, has kept pace with almost $1 billion in investments (
2
).
Our federal government has set the lofty goal of becoming a top-three global hub in just six years, and the Canadian life-science industry has joined forces, determined to gain that third spot. The country’s global life-science venture is adM...
www.istockphoto.com
Digitalization of manufacturing operations is a major challenge that many industries face. With the advent of smart equipment, automation of unit operations and complete processes, and digitalization of batch documentation, more data are generated now than ever before. The information must remain manageable, and data integrity needs to be ensured. The challenge for biomanufacturers will be to ensure that their entire large output of data will be attributable, legible, contemporaneous, original, and accurate (ALCOA) as defined by the US Food and Drug Administration (FDA) and other regulatory agencies.
Parallel to the digitalization efforts of the biopharmaceutical industry, a new technology for storing data in a decentralized database format was designed: blockchain. The concept has become known especially for cryptocurrencies such as Bitcoin, but it offers more possibilities. By using specific frameworks such as Hyperledger (
1
) that provide tools for development of blockchain-based a...
www.istockphoto.com
The biopharmaceutical industry uses living biological systems as a platform for manufacturing of protein-based drugs, vaccines, and other therapies derived from or consisting of different cell types. On one hand, living systems are inherently susceptible to viral infection and may harbor endogenous viruses, so the potential for such contamination cannot be eliminated. On the other hand, the industry has an excellent patient-safety record.
Viral safety is achieved through three fundamental measures: prevention (e.g., by selection), removal (by clearance and/or reduction), and detection (by testing). First, multiple preventive measures are used, including control and viral testing of raw materials (including media) ideally certified to be free of animal-derived components and use of unidirectional closed processes. Second, virus inactivation and removal steps are incorporated, and their effectiveness is confirmed through validation studies. For example, results must demonstrate the capab...
Sponsored Content
Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture: Using Functional Performance Indicators to Assess a Small‑Scale ModelImplementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture: Using Functional Performance Indicators to Assess a Small‑Scale Model
Figure 1a: Viable cell density (VCD) and viability of Chinese hamster ovary (CHO) cells cultured using cell settling in minibioreactors
Changes to bioprocessing in the biopharmaceutical industry are driven by the need for increased speed, lower cost of goods (CoG), and greater flexibility (
1
). To meet these challenges, the industry is adopting strategies that include intensified processing. During the initial stages of intensified processing, it is essential to identify the most productive and/or stable clones for use before starting pilot-scale studies. That requires screening large numbers of clones and then further testing the most promising ones in benchtop bioreactors. The need to perform large numbers of time-consuming, resource-intensive experiments has led to development of micro- and mini-scale bioreactor systems that offer a small-scale, high-throughput (HT) solution for accelerating clone/media selection and process development.
Figure 1b: Viable cell density (VCD) and viability of CHO cells ...
Bai-wei Gu
,
Juan Lagos
, and
Matthew Weaver
(heads of cell line development, upstream process development, and downstream process development groups, respectively, at
WuXi Advanced Therapies
, ATU) joined forces on 29 October 2019 to feature their company’s viral-vector manufacturing capabilities for cell and gene therapies. In addition to adherent platforms for lentivirus (LV) and adenoassociated virus (AAV) vectors, ATU soon will offer suspension-cultured viral vector platforms for them as well as analytical measures that support release testing. Transitioning from adherent to suspension processes required up- and downstream adaptation. Gu, Lagos, and Weaver shared what they learned about process development (PD) during that transition and detailed what challenges remain before their platforms can launch later in 2020.
The Presentation
Current Capabilities
: ATU’s work with suspension PD stems from success with its adherent viral vector platforms for AAV and LV. The former includes a conventional u...
Turn-key single-use aseptic sampling devices (ASDs) have diminished bioprocess contamination risks significantly. But depending on testing, facility, and storage needs, some ASD container types are more effective than others are.
Bobbi Allen
(technology expert at
Sartorius Stedim Biotech
North America, SSB) focused her 8 January 2020 “Ask the Expert” presentation on “what, why, when, and where” operators must sample aseptically from stainless-steel tanks. Using data from in-house testing of aseptic sampling containers, Allen offered key considerations for sterility, process monitoring, and endotoxin sampling.
Allen’s Presentation
Regulatory agencies require process sampling at the beginning or completion of significant manufacturing steps, and operators must withdraw samples without tainting them or their sources. Compared with needles and septa, valve-and-bottle assemblies, and steam-in-place (SIP) valves, turn-key ASDs have enhanced sampling significantly. ASDs are self-contained, and because they a...
Image: iStock/Grafner
Bioprinted organs soon could revolutionize clinical trials, transplantation, and regenerative medicine. But as Chris Lo reminds us in a
new GlobalData report
(
1
), several technical hurdles must be negotiated before biopharmaceutical companies can harness three-dimensional (3D) bioprinting for such purposes. BPI explores persistent printing problems and promising solutions below by analyzing Lo’s report alongside commentary from founding editorial advisory board member
Bill Whitford
(bioprocess strategic solutions leader at
GE Healthcare Life Sciences
),
Lev Gerlovin
(vice president in the
life sciences practice at Charles River Associates
, CRA), and
Andrew Thomson
and
Jack Vailas
(both associates at CRA).
Handy Hardware
Until recently, technical gaps made 3D bioprinting slow and inefficient. But new hardware is improving the handling of cellular substrates. Lo highlights Cellink’s BIO X6 printer (
2
) as a particularly promising model, noting that the technology “incor...