Although somewhat of an arbitrary practice, celebrating an anniversary milestone offers a chance for useful and productive reflections. Events of the past couple of years placed a number of projects on hold — especially some that we wanted to launch at in-person events. But finally, coming this month, we are introducing the BPI Best Places to Work in Biotech awards. In the midst of celebrating the publication’s 20-year anniversary, and given the current job market, we feel that this is an ideal time to highlight the companies that are getting it right. Which ones are best sustaining business while meeting the needs of (and attracting) new employees?
The 10 award categories are listed below, and soon you’ll see full details online. We will award the winning company in each category and identify the overall top-10 companies based on the final scores. Finalists will be announced in August, and we’ll celebrate the award winners in our September issue (and at the BWB event in Boston).
Sustainability:
To what...
In 2019, Expression Therapeutics (ET) obtained investigational new drug (IND) approval for its lead clinical product. The third-generation lentiviral vector (LV) expresses a bioengineered coagulation factor VIII to be used in an autologous hematopoietic stem- and progenitor-cell gene therapy for patients with severe hemophilia A. Like many other emerging biotechnology companies, ET’s initial strategy used reputable contract development and manufacturing organizations (CDMOs) for vector and cell manufacturing needs and a prominent clinical contract research organization (CRO) with extensive experience in early cell and gene therapy trials to support clinical development. However, we revisited the decision to do so based on our early experience and knowledge of discrepancies encountered by others in transitions from CDMO to internal manufacturing between phase 1–2 and phase 3. Challenges encountered in outsourcing included delays in securing viral vector manufacturing slots caused by capacity shortfalls, la...
Open-plan office area
(www.dpsgroupglobal.com)
Cell and gene therapies (CGTs) are progressing rapidly through development pipelines and advancing through clinical trial phases. Manufacturing capacity will need to be sufficient when such products are approved for commercialization. Thus, biomanufacturers are seeking ways to leverage multimodal facilities. I spoke with Stephen Judd, who is principal process engineer for biologics and cell and gene therapy at DPS Group, an engineering and construction management consultancy. We talked about design considerations for multimodal facilities, how such facilities contribute to overall sustainability efforts, and the advantages brought on by different construction approaches.
Our Discussion
What is a multimodal facility?
A
modality
refers to a specific type of manufacturing process or approach. Beyond clinical-sample processing, such approaches in CGT processing include viral and nonviral vector systems, which can be further subdivided into adenoassociated vir...
In an example of Ukrainian defiance, Enamine workers pose outside the company headquarters in Kyiv early in April 2020. (
https://enamine.net
).
The past few years have taught people around the world that “It can’t happen here” is never a good bet. From the COVID-19 pandemic to political upheavals to Russia’s invasion of Ukraine, we’re all experiencing a reality that would have seemed impossible not so long ago. And as numerous analysts and experts discuss on television and social media and in other publications what such events could mean politically and socially, we at BPI must maintain our focus on the biopharmaceutical industry and its associated ecosystems. With that in mind, I got in touch through mutual connections with a company in Kyiv, Ukraine, to discuss the realities of getting work done in a country that is literally under fire.
Vadim Klyushnichenko (vice president of pharmaceutical development and quality for the Calibr nonprofit translational research institute at Scripps Research in La Jol...
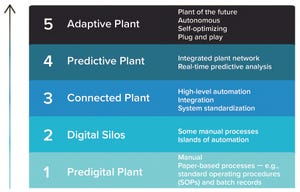
Pharma 4.0 technologies, offshoots from the Industry 4.0 model, focus on introducing new technologies for increased levels of digitalization within the pharmaceutical manufacturing industry. Many companies hesitate to embrace the Pharma 4.0 concept fully even though digitalization efforts are leading the way toward new levels of efficiency and productivity. The biopharmaceutical industry currently lags behind other industries in implementing digital technologies because of its rigorous and strict regulatory requirements.
Adopting Pharma 4.0 tools and concepts benefits manufacturers by harmonizing the flow of R&D VR data through manufacturing and distribution, strengthening cybersecurity, and improving quality while maintaining regulatory compliance. Technologies that help pharmaceutical manufacturers find new, innovative avenues to realize higher levels of connectivity and automation include artificial intelligence (AI), augmented reality (AR), virtual reality (VR), and digital twins.
Figure 1:
Digital p...
As BioPhorum authors stated in 2019, “It should come as no surprise to anyone familiar with biomanufacturing that current designs of bioprocess facilities as well as associated manufacturing spaces and support operations require excessive amounts of manual labor and manual interventions that lead to high labor costs and, consequently, total cost to supply” (
1
). Back then, a realization was starting to take hold in the biopharmaceutical industry that modern robotics showed great potential. However, a cohesive vision of their future in biotechnology was not yet formed. It has taken three years for this vision to develop into the road map described herein.
The Common Milestones
The road map culminates in the possibility of a fully automated cell and gene therapy facility by 2030. These milestones apply to most biomanufacturing facilities and will need to be tacked on the road towards full automation.
If we consider automation to be the doorway to lights-out facilities of the future, then consumables (e.g.,...
Pressure vessels are enclosed containers used to contain liquids, vapors, and gases at pressures that are significantly higher or lower than the ambient pressure of their surroundings. Equipment such as bioreactors, holding tanks, mixing tanks, separators, and heat exchangers all are examples of pressure vessels. As such, they form an integral part of biopharmaceutical manufacturing. Apart from pressure containment itself, a key challenge in building pressure vessels is making them meet the high purity and cleanability requirements of bioprocessing.
As single-use technologies have been integrated into more bioprocess operations over the past couple of decades, their suppliers have addressed many associated concerns such as those related to scale and leachables/extractables, as well as supply-chain and waste questions that arose with expanded use of disposables. Even the gassing rate, mixing power, and heat removal needs of microbial fermentation applications are beginning to be met now by some single-use ...
Messenger RNA (mRNA) emerged as a powerful therapeutic tool for treatments in gene therapy, oncology, and infectious diseases, as recently demonstrated by vaccines against Covid-19. mRNA is produced by an enzymatic reaction that can be rapidly designed and scaled-up, and the platform is highly adaptable to different targets. One of the greatest challenges in mRNA production is the removal of process-related impurities stemming from in vitro transcription (IVT) reaction, such as residual nucleotide triphosphates, DNA template, enzymes, abortive transcripts.
Affinity-based chromatographic isolation of mRNA is robust and simple, lending itself as a useful industrial platform. mRNA constructs typically contain a 3’ polyA tail to increase stability in vivo, thereby enabling affinity purification using oligo-deoxythymidinic acid (Oligo dT) probes covalently coupled to a solid support. Macro-porous polymethacrylate monoliths offer high binding capacity and resolution for mRNA due to the convective nature of inte...
Over the past few decades, the biotechnology industry has brought to patients a medical revolution with the most advanced medicine ever seen. Yet much of the world’s population cannot afford or get access to these breakthrough therapeutics. That is in part a consequence of the high associated costs of development and biomanufacturing, extended times for regulatory review and approval, and a lack of regional manufacturing and dependable supply chains (because of facility costs and a scarcity of expertise).
The recent unprecedented success in developing and delivering into the arms of patients a collection of safe and efficacious vaccines and treatments against COVID-19 was made possible through relentless effort and collaboration among all industry stakeholders: drug developers and manufacturers, regulators, governments, vendors, suppliers, and global health partners. However, access to those life-saving treatments has been slow or unattainable for many geographies with vulnerabilities in their supply chai...
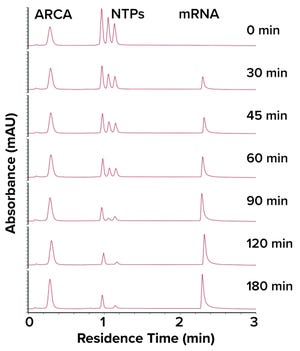
Figure 1:
Chromatogram from analysis of an IVT reaction using a CIMac PrimaS monolith chromatography column.
In vitro transcription (IVT) is a critical step in messenger RNA (mRNA) production. In a March 2022 webinar, Rok Sekirnik (head of process development for mRNA and plasmid DNA (pDNA) applications at BIA Separations, part of Sartorius) explained that optimizing concentrations of reagents used during IVT helps to maximize the amount of mRNA that is produced. Drug developers have great need for analytical methods that can measure IVT in real time. Sekirnik showed how CIMac PrimaS monolith chromatography columns enable at-line analysis of IVT, including concentration monitoring of nucleoside triphosphates (NTPs), capping reagents, and plasmids.
The Presentation
Sekirnik noted that a 4,000-nucleotide mRNA molecule such as that featured in mRNA vaccines against SARS-CoV-2 is
~
1.3 MDa — approximately 10× larger than a standard IgG antibody. Messenger RNA also carries a negative charge and becomes hydro...
Critical quality attributes (CQAs) must be characterized thoroughly to establish a biopharmaceutical’s potency, efficacy, and safety. Such characterization involves some of the most difficult analytical activities that are performed in a biologic’s product life cycle. There is great need for analytical tools that can facilitate such studies. In March 2022, Dr. Kalhari Silva (head of scientific research at Custom Biologics) and Dr. Bob Dass (senior scientist at Sartorius) joined BPI to describe how Octet biolayer interferometry (BLI) technology can be applied to analyze biomolecular interactions. Dass provided an overview of its capabilities. Silva explained how Custom Biologics, a contract research organization (CRO), uses the technology to design and optimize assays of antibody (Ab) binding kinetics.
Basic Principles
Dass described the Octet BLI technology as a high-throughput platform for label-free, real-time detection and evaluation of biomolecular interactions. The system dips a row of coated fiberop...
In 2018, Synlogic explored options for producing clinical-trial material for its lead programs, including a candidate therapy for the rare metabolic disease phenylketonuria (PKU). Like other emerging drug developers, the company evaluated the merits of outsourcing manufacturing to third parties. However, Synlogic leverages synthetic biology tools to design and develop therapeutics based on genetically engineered microbes. Thus, it also needed to consider requirements specific to live biotherapeutics. Late in 2018, Synlogic announced plans to establish its own current good manufacturing practice (CGMP)-compliant infrastructure for internal production of therapeutic candidates.
Despite obstacles raised by the COVID-19 pandemic, Synlogic has expanded its capabilities and advanced clinical candidates. In April 2022, I corresponded with Tony Awad, the company’s chief operating officer, to learn about manufacturing requirements for live biotherapeutics and considerations for developing internal capabilities.
Ou...