January-February 2021
With this issue, BPI celebrates its 19th birthday.
I have pretty much run out of words to talk about the year we all just endured and the changes we’ve had to make in our lives to sustain our families, businesses, and overall mental and physical health. We editors want to respond appropriately to the complex issues currently facing the biopharmaceutical industry. But with so many technologies advancing so quickly, it’s difficult for a (mostly) monthly publication such as ours to keep up. Our publication timelines are shorter than those of academic journals: three months at minimum from submission, to review, to publication — depending on available space. But what may be breaking news in January when a manuscript is accepted for a March or April issue could be superseded by publication time. We had to keep reminding ourselves of this as we planned our 2021 editorial calendar. It lists only short labels for themes, mainly, to give contributors room to address what is truly cutting edge.
Here, then, are some...
In the fall of 2020, BPI premiered its Readers’ Choice Awards program. Concentrating on articles published from September 2019 through June 2020, with rankings based on digital engagement, BPI staff identified the most popular articles within each of six categories of bioprocess coverage. You ranked those articles in terms of innovativeness, applicability, and presentation. We’re pleased with the turnout and delighted now to congratulate the winners, who were
announced first online
.
Analytical Award
BPI congratulates the winner of our
Readers’ Choice Award for analytical articles
this year,
Veeren M. Chauhan
(University of Nottingham, UK). In “Fluorescent Nanosensors: Real-Time Biochemical Measurement for Cell and Gene Therapies” from our May 2020 featured report on manufacturing 4.0, Chauhan introduced tools that permit real-time analytics of subcellular biochemical processes. These could play a significant role in the optimization of CGT manufacturing and facilitate the transition of such “advance...
The biopharmaceutical industry continues to develop advanced manufacturing processes, systems, technologies, and facilities. Regulations play a significant role in assessing and approving marketing authorizations for drug products that are submitted for approval. The industry and regulators together should form a coordinated, streamlined process that delivers much-needed medicines around the world.
Global Complications
The complex global nature of biopharmaceuticals sometimes means that progress is not always as smooth as it could be. One main reason is that regulatory agencies often differ in their degrees of harmonization. That has created an environment of divergence, in which countries each have unique standards, requirements, submissions, and review processes.
Some agencies make a few changes to US or European submissions. Typically, those receiving agencies take review and approval reports from more-established agencies and approve or reject submitted files based on those reports. However, other age...
In
BPI’s June 2020 issue, the first installment of this series
introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (
1
). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the current pandemic, SU systems are instrumental to developing new drugs and vaccines quickly and safely.
Below we outline current thinking on how to design materials, systems, and processes to support the “rethink, reengineer, reduce, reuse, and recycle” paradigm of the circular-economy concept for plastic and packaging. Through engineering efforts to improve sustainability, SU technology will become an even better manufacturing technology option in the future. In an u...
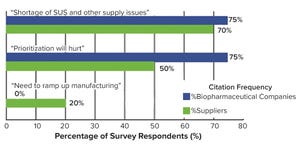
Figure 1: Top-level near-term concerns brought about by the COVID-19 pandemic (
2
)
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (
1
). Top trends from this report are highlighted below.
Industry Growth Continues
The biopharmaceutical industry and its bioprocessing sector are healthy and continue to expand in revenue, breadth, importance, and diversity. Worldwide sales of biopharmaceuticals are approaching US$300 million and increasing globally at ~12% annually, with supporting growth in bioprocessing markets (supplies and services) ...
The motto of the European Serum Products Association (ESPA) — “Serum Saves Lives” — reaffirms the essential role that animal serum plays in cell-culture–based research and applications to protect the health of both human and animal populations. Animal serum, especially fetal bovine serum (FBS), needs to be available in abundant supply and at affordable prices to medical and veterinary facilities all over the world. With one billion cattle globally, the supply of FBS should be plentiful, but not all countries qualify as producers for the international market.
The animal-health status and regulatory infrastructure of countries differ around the world, as does their ability to control and eradicate diseases. Those factors are influenced by the size of the country, its infrastructure of roads and communication, bordering countries, the cattle population and average herd size, and the number of veterinarians employed in the public sector — among other things. To be approved as FBS suppliers, exporting countrie...
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in
Escherichia coli
through fermentation (
1, 2
). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of
E. coli
, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm for correct folding (
3, 4
).
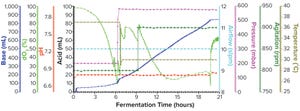
Figure 1a: Fermentation profile of a Sartorius Biostat ED system (15 L) using one temperature shift from 37 °C to 30 °C at the 6.5-h point; fermentation parameters that could be actively controlled during growth are shown on the right
y
-axes (pressure, airflow, agitation, and temperature), and parameters that were monitored during growth are shown on the left
y
-axes (dO
2
, pH, an...
Aggregation is a common cause of protein instability, which renders a biologic product unfit for therapeutic use. Sometimes it is difficult to purify monomeric proteins from oligomers because of similarities in their isoelectric points (pIs). Proteins such as hormones have pI ranges similar to their oligomers and thus can be difficult to separate out using a conventional polishing chromatographic step such as ion exchange. With those pI similarities, removal of oligomers to a considerable extent by ion exchangers can compromise protein recovery, thus causing unacceptable product loss during processing.
To minimize the yield loss during extensive reduction of oligomers, we considered the use of novel techniques with process optimization. A downstream process should be robust and reproducible at scale-up with minimum impact on recovery. Much research is ongoing in this area, with limited successes in purifying proteins from oligomers. Thus, techniques are needed that can reduce oligomers to well below accep...
Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and CapacityEvaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
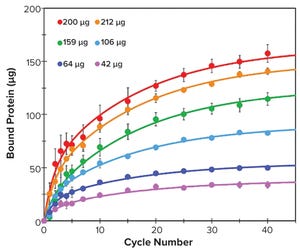
Figure 1: Binding kinetics of different amounts of human IgM to resin M over 40 binding cycles; bound IgM was measured at 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, 30, 35, and 40 binding cycles (filled circles). Data were fitted with a double exponential function (solid lines).
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (
1
). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (
1–3
). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX) chromatography can require additional polishing steps (
4
). Recent introduction of IgM-affinity ligands has enabled single-step affinity purification, thereby opening therapeutic application oppo...
A fully integrated bioreactor system with PAT sensing and precision glucose control for CHO cell culture, the BR1000 advanced-control bioreactor system from Yokogawa Electric Corporation handles 1-L to 5-L stirred-tank bioreactor vessels.
www.yokogawa.com
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with real-time integrated monitoring and advanced analytics that can enable automation, drive performance, and improve data-rich quality control. However, there are multiple options for sensors and technologies that monitor important cell-culture variables or critical process parameters (CPPs). Furthermore, cell culture vessels can be disposable single-use bioreactors (SUB) or reusable glass or stainless-steel models. They can operate in stirred tanks, with rocking platforms (bags), or in perfusion configurations. The sensors and monitoring technologies selected for these configurations must be suitably designed an...
https://www.dupont.com
Adoption of single-use systems (SUS) has increased steadily over the past few years driven by an increased focus on manufacturing biologics that require smaller scales than blockbuster drugs. Although SUS provide many benefits, some factors need to be considered when implementing them in biopharmaceutical production. With the regulatory landscape intensifying worldwide, drug manufacturers are expecting more data from suppliers to support the purity claims of supplied products, especially related to the purity of their product-contact surfaces.
In this article, we discuss how DuPont Liveo Pharma Tubing products meet the very high level of purity required in the biopharmaceutical industry:
Learn more on how DuPont’s silicone tubing products can meet the highest reliability, purity, and safety in critical biopharmaceutical processes.
Fill out the form below to read the complete article now.
Lise Tan-Sien-Hee,
PhD, is a technical service and development specialist for healthcare, DuPon...
Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA and reduce, restore, or modify protein expression through several distinct mechanisms. ASO technology has become an important drug discovery platform for most major pharmaceutical companies. To date, six antisense drugs have been approved by regulatory agencies to treat diseases spanning viral infections, hyperlipidemias, and neurological diseases. More than 50 additional ASO drugs are in clinical trials.
For an ASO drug product, an assay of its active pharmaceutical ingredient (API) provides a critical quality attribute (CQA) because of its direct impact on drug safety and efficacy. Therefore, proper in-process control (IPC) measurements must be implemented to ensure that an ASO assay results are within an acceptable range during different unit operations of drug-product manufacturing (e.g., API compounding and bulk filtration). We have used a platform HPLC-UV method for IPC assay measurements...
Chromatogram review enables evaluation of packed-bed chromatography processes. Typically, that method entails paper-based, qualitative comparison of a profile obtained during a run with another from a reference batch. Such a protocol can be time consuming, resource intensive, and susceptible to operator error.
Martin D. Jensen
(senior engineer at Fujifilm Diosynth Biotechnologies, FDB) delivered an “Ask the Expert” presentation on 20 October 2020 to describe advantages that his company gained by transitioning from paper-based to digital chromatogram review.
Jensen’s Presentation
Chromatogram review serves as an important in-process control because it can identify cases of poor column packing, resin degradation, and equipment malfunction. For instance, excursions of column outlet conductivity can represent channeling and dead-volume formation. Atypical transitions in an impurity’s UV absorbance can indicate changes in its interaction with a resin (e.g., nonspecific binding). Unexpected interactions betwe...
Upstream operations can benefit significantly from real-time, in-line testing of cell-culture conditions. Yet as
Jake Boy
(senior application scientist at Scientific Bioprocessing Inc., SBI) observed in his 5 November 2020 “Ask the Expert” webcast, traditional electrochemical probe sensors do not perform well in T-flasks, shake flasks, Petri dishes, and microfluidics devices. Boy described how fluorescence-based optical sensors can glean valuable data from small culture devices, assisting with research into cellular growth.
SBI’s Presentation
Boy began by highlighting successful applications of optical sensors in small culture devices. In one case, SBI worked with researchers at Virginia Commonwealth University to monitor rat chondrocyte growth in Petri dishes incubated with CO
2
. Optical sensors enabled real-time pH monitoring in those studies. SBI also partnered with German startup company aquila biolabs, adding optical sensors to its biomass monitoring systems for shake flasks. The teams tested that...
The Opto CLD workflow provides a complete visual record from single-cell cloning into NanoPen chambers through recovery of selected clones into well plates. (
https://www.berkeleylights.com/workflows/cell-line-development
)
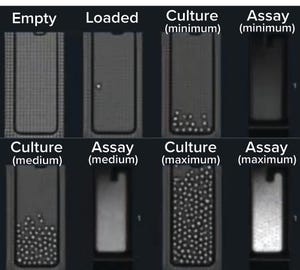
On 1 December 2020, BPI presented an “Ask the Expert” webinar with
Tanner Nevill
(vice president of program management at Berkeley Lights). Monoclonality assurance is a central regulatory requirement for all cell lines producing biologic therapies. Well-plate imaging is the “gold standard” for confirming that a cell line originates from a single cell, but it is labor intensive and prone to errors in the form of “ghost cells” and debris that look like cells. Using the Berkeley Lights Beacon system, cells are cloned, cultured, and assayed over multiple days directly on a microfluidic chip. The system provides a complete visual record from initial single-cell cloning through recovery of selected clones into well plates. Cell lines are recovered with >99% monoclonality assurance in under...
Biopharmaceutical companies have demonstrated a rapid and powerful response to the COVID-19 pandemic, with innovators leading the way. A rush of investor money has been pouring into companies with the best odds of making it to the vaccine finish line. But such heavy investments in one sector lead some industry experts to wonder about the financial support of research into cure pathways for other serious diseases such as cancer, multiple sclerosis, and Alzheimer’s disease. When the pandemic abates with vaccines and optimal treatments in place, other diseases still will be robbing patients’ lives and diminishing their quality of life. How bumpy is the road for biopharmaceutical developers working on breakthroughs for non-COVID-19 diseases? Below, I’ve outlined what such companies are wrestling with during this time.
Access to Capital:
According to the BioCentury’s BCIQ database, US$18.8 billion was raised in 2019 for global biotechnology ventures, up from $17 billion in 2018. Although there’s been a fear o...
Figure 1:
Metrics obtained from different downstream process samples
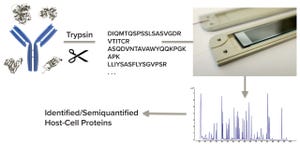
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely.
Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high throughput, sensitivity, and selectivity. Polyclonal antibodies used in the test typically are generated by immunizing animals with an appropriate preparation derived from production cells without the product-coding gene. But ELISAs do not recognize all HCP species completely — they cannot detect HCPs to w...