Single-use technology has been transforming the modern biomanufacturing workflow. You can now find single-use bags at every stage of bioprocessing: bioreactor and mixing systems, harvest and collection, purification, liquid and powder storage, and transportation.
The Strength of Simplicity: One Film for Bioprocessing
At GE, we’re introducing one film for our entire portfolio of single-use systems. It’s named Fortem after the Latin word for
strong
— and aptly so because it is strong in many ways. It was born of customer collaboration, supplier partnership, and GE’s bioprocessing expertise. It offers the simplicity of qualifying one film across the full range of your single-use bioprocessing systems, saving you time, effort, and cost.
Unlike other platform films, Fortem film is not a retrofitted solution. It has been designed from the ground up for bioprocessing. To achieve this, we’ve partnered with Sealed Air Corporation, a manufacturer of primary films for pharmaceutical solutions. We’ve listened close...
MabPlex provides world-class solutions to biopharmaceutical clients around the world. Our company is based on the quality by design (QbD) principle, from site construction to manufacturing and delivery of our clients’ biologic drugs. With an expert team adept in both chemistry and biology, MabPlex provides global contract development and manufacturing organization (CDMO) services in biopharmaceuticals. Capabilities span the scope from monoclonal antibodies (MAbs) and fusion proteins to the most complicated antibody–drug conjugates (ADCs). Our 275,000-ft² manufacturing space offers flexibility and complete services from upstream cell line development to manufacturing and fill–finish of commercial products. Our technical expertise allows us to provide effective high-quality solutions for your biomanufacturing needs. With that, MabPlex delivers our commitment to you through our quality manufacturing, meeting your expectations.
Login to read the full PDF of the application note.
Rentschler Biotechnologie GmbH, located in Laupheim, Germany, is a leading biopharmaceutical contract development and manufacturing organization (CDMO) for bioprocess development and manufacturing of biopharmaceuticals and an experienced specialist in mammalian cell culture. The company’s clients include innovative biotech companies and major pharmaceutical companies around the world. Many years of experience and excellence in finding solutions as well as certified quality management and advanced technologies ensure Rentschler’s high-quality standards. Rentschler Biotechnologie is a family-owned company employing approximately 700 people.
Full-Service Provider from Gene to Vial:
Rentschler’s full-service offering includes all activities from bioprocess development through to current good manufacturing practice (CGMP) manufacturing, analytical testing, fill and finish, and all associated consulting activities in terms of project management and regulatory support.
Login to read the full PDF of the applicat...
Richter-Helm is a Hamburg, Germany–based contract development and manufacturing company with a proven 25-year track record, specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 160-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies (European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), an...
Wacker Biotech is “The Microbial CMO” — the partner of choice for contract manufacturing of therapeutic proteins using microbial hosts. Our service portfolio covers molecular biology, process and analytical development, and the GMP production of biologics for clinical trials and commercial supply. Founded in 1999 as a spin-off from the Hans-Knöll Institute in Jena, we are a 100% subsidiary of Wacker Chemie AG since 2005.
The sites in Jena and Halle provide a complete range of services for the development and GMP-compliant manufacture of biopharmaceuticals. Two manufacturing lines with 300-L and 1,500-L fermentation vessels and matching DSP scales are available to suit all possible customers’ needs.
WACKER holds biomanufacturing certificates from the relevant authorities for both sites and follows the ICH Q7A guidelines for GMP-compliant production of biologics. The facility in Halle has been EMA and FDA approved for the commercial production of Reteplase (trade names Rapilysin® and Retavase®), a thromboly...
Sponsored Content
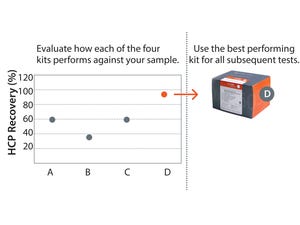
Removal of host cell protein (HCP) impurities in drug substances is critical in the manufacture of highquality drug products and for patient safety. A key requirement is a thorough analysis of HCP contaminants, which may vary considerably depending on cell line, media, and process parameters. How well a particular HCP assay recognizes all HCPs will depend on how well its antibodies match the actual HCP composition, their abundance, and affinities for each HCP. Accordingly, various commercial generic HCP ELISAs may differ substantially in their ability to detect similar types and levels of HCPs.
Platform or specific HCP assays offer peace of mind. However, because of the high risk of drug failure, such assays are a worthwhile investment only in later stages of development.
Login to read the full PDF of the application note.
Sulfur is an essential component of all living cells. In plants and animals, the amino acids cysteine and methionine contain most of the sulfur, and the element is present in all polypeptides, proteins, and enzymes that contain these amino acids. Sulfur-containing compounds often show different biological activities and provide important functions in pharmaceutical industry applications. Sulfonamides, thioethers, sulfones, and penicillins are the most common scaffolds in sulfur-containing drugs. Sulfonamide drugs were the first antibiotics to be used systemically, and they paved the way for the antibiotic revolution in medicine. Sulfonamides function as diuretics and in hypertensive therapy. Sulfones are found in numerous medicines. Their main functions are as proton pump inhibitors and antibiotics. Penicillins are a group of antibiotics that were among the first medications to be effective against many bacterial infections caused by
Staphylococcus
and
Streptococcus
species. Penicillins are still wide...
The biological properties of IgM antibodies make them very effective vehicles for in vitro diagnostics and therapeutics. However, purification of IgM antibodies is far more complex than that for IgG antibodies. Furthermore, affinity chromatography is not ideal for purifying IgMs due to the required elution conditions. Here we propose a nonaffinity-based platform for IgM purification. This strategy utilizes a cation exchange (CEX) media, Nuvia™ S, for capture, and a mixed-mode media, CHT™, for polish purification. It is suitable for purification of neutral and basic IgMs. We optimized the protocol with three different IgMs and present reduced PAGE images of the purified samples. This is an ongoing study and further optimization will be needed for other IgM molecules.
Impurities such as viruses are typically present during the manufacturing of biological drugs. The viruses may be present in the unprocessed material from the upstream collected harvest, such as released from cell culture media (endogenous) or accidentally introduced during processing (adventitious).
Viral clearance is required by the FDA and international regulatory bodies as specified in the ICH Q5A documents.
In this study we evaluated two chromatography steps in the purification of a monoclonal antibody (MAb) for viral clearance. Evaluation was performed with four model viruses commonly used for the testing of MAb products.
Studies were performed as spike/chase experiments, where a known quantity of virus is added to unprocessed material, and remaining virus is quantitated following processing. A schematic for chromatography step testing is shown in the Methods – General section.
Login to view full poster.
Critical objectives for the biopharmaceutical industry are the creation of robust, reproducible processes which result in consistent critical product quality attributes and yields. To meet these requirements within a short time period it is important to apply platform production processes which consist of a common host cell line, expression vector, cell line development process, cell culture media/feed, process control and scale-up methodologies during cell line development, process characterization and cGMP manufacturing.
In this study we integrate mathematical based approaches with system characterization studies to refine important cell culture parameters for multiple cell lines and recombinant monoclonal antibodies to identify process parameters which underpin robust platform production processes.
Bacteria and their byproducts can negatively affect the safety and potency of a biopharmaceutical drug. At a minimum, bioburden contaminations lead to reduced productivity as a result of lost batches and/or deviation investigations. Striving towards a bioburden-free process, biopharmaceutical companies and their suppliers must collaborate. To meet this challenge, GE Healthcare is committed to continuous improvement of its chromatography resins to enable delivery of products without detectable bioburden. For example, development of Protein A resins to withstand high NaOH concentrations and investigations of an oxidizing agent for sanitization are conducted.
The binding mechanism between the engineered C domain of the Amsphere™ A3 protein A (PrA) ligand and a VHH single domain antibody (sdAb) was revealed. Binding sites in the PrA ligand in helices 2 and 3 and in framework regions 1 and 3 of the VHH were confirmed. Identified VHH residues are not involved in antigen recognition. Overlap with a human VH showed the same interaction sites. These results provide insight on why Amsphere A3 is a suitable tool for antibody fragment purification, with the known benefits of high process robustness, selectivity and caustic stability. Amsphere A3 has the same capacity for sdAbs as the current non-PrA affinity ligand resins (20-30 g/L).
Login to view full poster.
The pipelines of biopharmaceutical companies are becoming increasingly diverse while improvements in cell lines are leading to more productive bioprocesses. These factors are driving new capacity demands for bio-pharmaceutical companies and their CDMO partners. It is becoming increasingly important, therefore, that companies implement innovative, highly efficient, flexible facilities that are capable of meeting these challenging capacity demands.
Login to view full PDF version of poster.
Abstract
Single-use bioprocess technology offers several advantages for manufacturing biopharmaceuticals, such as increased transportability of fluids throughout the bioprocess workflow and a greater diversity of systems to support specific unit operations, e.g., rocking bioreactors. However, due to the flexible nature of the plastic materials used to construct the single-use containers, the flexural properties of the bioprocess film are critical for performance in such applications. This document focuses on how the resin selection and architecture of a bioprocess film can be optimized to maintain critical performance attributes, such as container integrity and gas barrier properties, under the significant forces during bulk liquid transportation and WAVE Bioreactor™ system applications.
Background
Single-use bioprocess technology is becoming more mainsteam in the biopharmaceutical industry. However, there are a number of challenges that still remain, and system requirements continue to evolve. Over the p...