There’s a funny meme going round social media about the naivete of conspiracy theorists: “My gut is that most of them have never been project managers. Their optimism is adorable.” And the adage may be that “too many cooks spoil the broth,” but I think it’s more like “too many stakeholders can overcomplicate anything.” That can apply to small teams working on relatively simple projects (a group of editors working on a single BPI issue, for example) as much as to the whole biopharmaceutical industry and the regulatory, commercial, and technical/scientific ecosystem that surrounds it. Sometimes it’s a wonder that any of us in publishing or drug development manages to accomplish anything on a given day. And yet we do.
Consider the European Union — a timely example right now if ever there was one. I’ve never imagined it could move as fast as it has in response to Russia’s invasion of Ukraine. Once-reluctant would-be member states are even getting fast-tracked for entry by a system that otherwise
seems
to mo...
HTTPS://WWW.ISTOCKPHOTO.COM
Significant growth of the cell and gene therapy (CGT) pipeline in recent years demonstrates the enormous potential of these modalities to treat or even cure otherwise intractable diseases. Several CGT products have been approved for clinical use over the past five years. More than 75 such products have come to the market around the world so far. They include chimeric antigen receptor (CAR) T-cell therapies that involve genetic engineering of patient cells ex vivo as well as in vivo gene therapies that introduce healthy copies of missing or faulty genes to patients through viral vectors.
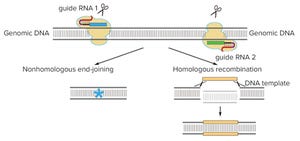
CGT technology development is advancing rapidly. Efforts are under way to unlock the potential of gene-editing tools such as the Nobel Prize–winning clustered and regularly interspaced short palindromic repeats (CRISPR) technology, to implement nanomaterials for gene delivery (in place of viral vectors), and to develop off-the-shelf allogeneic therapies. As a result, an estimated ~1,200 clinical...
At Pluristem in Haifa, Israel, attachment-dependent cells are cultured on plastic discs in bioreactors for large-scale stem cell production.
Propelled by year after year of record-setting investments and regulatory approvals, cell and gene therapy (CGT) innovators are on track to revolutionize medicine by providing potential cures for many conditions. Now, CGT manufacturers must plan for a future beyond the production constraints of laboratories. What needs to happen now to prepare for a sustainable, commercially viable scale-up process in years to come?
To answer that and other important questions about CGT production, my company, the CRB Group, surveyed more than 500 leaders from the life-sciences industry and documented their perspectives in the new
Horizons: Life Sciences
report (
1
). Nearly half of those surveyed reported that their companies have or plan to include CGT products in their pipelines. The respondents’ insights reveal just how far the field has come in the past decade and indicate wha...
In many ways, cells are the ultimate therapeutic product. They can integrate and participate in different biologic processes and replace missing biological functions. Cell therapies are dynamic, versatile, and with the appropriate engineering, capable of influencing and correcting disease processes robustly. Cell therapies essentially are living medicines, and their adaptability contrasts with conventional drug modalities that generally have only a single specific target or effect.
Because cell therapies are highly complex modalities, their scientific and R&D challenges are different from those of other biopharmaceuticals. No “template” approach for cell therapy production exists, and biomanufacturers cannot adapt technologies directly from small-molecule or antibody manufacturing workflows. A cell therapy manufacturing process is connected integrally to early development steps such as product design and to downstream operations such as formulation and cryopreservation.
New technologies for streamlining p...
The product development team at a gene therapy contract development and manufacturing organization (CDMO) was working on a high-priority drug-substance project for a key client. The material was crucial to that client’s early stage clinical trial, with an immediate value over US$500,000 to both the client and the CDMO. Unfortunately, the bioreactor used in the upstream process — a transfection unit operation for an adenoassociated virus (AAV) vector — had developed an intermittent problem that could force it to shut down, killing the cultured cells.
To meet the clinical trial timeline, the production run had to move forward, and no other bioreactor was available. If the process scientist and technicians could catch the problem in time when it occurred, then they could diagnose it quickly and reset the unit with no ill effects on the cells. But the lead scientist could not be there 24 hours/day, seven days/week over the course of the multiple-day culture.
The CDMO’s automation-engineering team accelerated ...
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (
1
), and the scientific community has issued equally high praise for the technology’s power and potential implications. These factors have made the outcomes of the interference cases fraught with economic benefits for the eventual victors — for both the scientists and the assignees of CRISPR-associated patents and applications. (
Note
: Just before this article went to press, the Patent Trial and Appeal Board [PTAB] ruled that between inventors from the Broad Institute and the University of California/Berkeley [an...
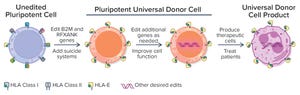
New treatments and approaches for tackling serious diseases are being developed using cell and gene therapy (CGT). CGTs are adding a new dimension to the way patients with such conditions can be treated in modern healthcare. But these advanced treatments require complex research, development, and manufacturing processes.
CGTs are related and to a degree interchangeable. However, gene therapies typically use some kind of genetic delivery system, (e.g., virus-based vectors) to treat a disease by replacing a malfunctioning gene or introducing a new gene into a patient’s body to alleviate a disease condition. Although cell therapy works in a similar manner, it usually involves modifying cells — whether from a patient or healthy donors — outside a patient’s body and then delivering those modified cells to a patient’s body to treat the disease.
Developing Partnerships
Based on 20 years of experience in the CGT field, I believe that one of the biggest challenges for these treatments comes in ensuring safety of t...
Advanced therapy medicinal products (ATMPs) hold much potential for improving healthcare. They offer hope for treating or even curing patients. The biopharmaceutical industry has recognized the importance of making such therapies accessible to as many people as possible. To provide personalized ATMPs, biomanufacturers are shifting toward flexible, patient-centered production processes.
A Paradigm Shift in ATMP Manufacturing
Ex vivo cell and gene therapies are particularly promising approaches to personalized regenerative medicine. Thus, it is no surprise that the numbers of US Food and Drug Administration (FDA) approvals for cell and gene therapies are rising for a broader swath of indications. By implication, the number of eligible patients is increasing. Significant interest in ATMPs from both patients and biotechnology companies is driving the biopharmaceutical industry to create an infrastructure for sustainable supply of raw materials and process components used in ATMP manufacturing.
Figure 1:
A ty...
The cell and gene therapy (CGT) industry has grown out of disparate groups of developers, suppliers, and service providers. Players in the sector must collaborate if we expect to commercialize new therapies at scale. Despite concerted efforts in the field, however, the critical question of how to automate CGT production has yet to be answered. Without automated processes in place, the sector risks spiraling costs and operational inefficiencies that could inhibit patient access to much-needed therapies.
Understanding such concerns, several institutions and companies — including my own — have joined the Cell and Gene Therapy Catapult’s Process Analytic Technology (PAT) Consortium. It aims to increase efficiencies and lower overall production costs by assessing applications and combinations of multiple technologies for process analytics within CGT manufacturing. Currently, this is the largest global consortium in the CGT sector, with more than 20 member organizations (
1
).
Initial Projects
Terumo Blood and ...
In-line process analytical technologies (PATs) hold much promise for enhancing control of biopharmaceutical manufacturing processes and, in turn, increasing process quality and reproducibility. During a January 2022 presentation, Ramsey Shanbaky (associate director of bioprocess applications at Repligen) described how his company’s CTech FlowVPX system for in-line protein concentration measurement enhances monitoring of downstream processes.
Shanbaky’s Presentation
Scientists often use ultraviolet–visible spectroscopy (UV-vis) to measure protein concentration in a drug substance (DS) or product (DP). Most UV-vis instruments determine that by measuring
absorbance
— defined in the Beer–Lambert law as the molar attenuation coefficient (
ε
) × species concentration (
c
) × optical pathlength (
l
) — across a fixed pathlength, treating
c
as a variable. But that approach yields only one measurement, and samples must be diluted to ensure that
c
values fall within an instrument’s linear range.
The FlowVPX s...