Strategies for Implementing a BPC in Commercial Biologics Manufacturing
November 1, 2009
Biopharmaceutical manufacturers use a range of bioprocess containers (BPCs) during the production and storage of biopharmaceuticals. Plastic bags, bottles, flasks, and carboys are all types commonly used in bioprocessing (1). A suitable BPC must be able to maintain aseptic integrity and be constructed of materials that will not harm product efficacy and/or purity.
The trend of “single-use” or “disposable” BPC technologies in the biopharmaceutical industry enables greater flexibility and better use of production facilities that are increasingly designed for multiple products (2). In some areas of biopharmaceutical manufacturing (e.g., cell culture), the shift has already become institutionalized, and single-use BPCs such as flasks, roller bottles, and spinners are in wide use (3). The paradigm is not yet as pervasive to the rest of biomanufacturing processes, but a growing trend is evident from the large number of conferences devoted to single-use technologies.
PRODUCT FOCUS: BIOLOGICS
PROCESS FOCUS: PROCESS dEVELOPMENT
WHO SHOULD READ: QA/QC, PROCESSDEVELOPMENT, MANUFACTURING
KEYWORDS: DISPOSABLES, VALIDATION, ANALYTICAL METHODS, DOCUMENTATION
LEVEL: INTERMEDIATE
Disposable BPCs are becoming available for a wider range of applications and process steps. As biopharmaceutical manufacturers experience the benefits of implementing single-use technologies, the added benefit of expanding their use into other upstream and downstream steps is increasingly apparent. To implement these systems in any area of biopharmaceutical manufacturing, a robust selection process must be in place to ensure that they are available at your supply requirements and adequate for the intended use. It must also provide assurance that a biologic product will not suffer from contact with and storage within a given BPC. Here we present an end-user perspective on what it takes to incorporate a new BPC in a manufacturing process for a commercial product.
Complete validation packages need to be in place for all BPCs used in a manufacturing process by the time of licensure filing. Such a package typically includes a combination of vendor and end-user tests, depending on the application and degree of exposure to the final product. Selection of a new BPC requires critical assessment of vendor-supplied information to evaluate suitability of the item for its intended use. Preimplementation evaluation will require some end-user testing to supplement information supplied by the vendor. A number of stakeholders are likely to be involved in implementing a BPC into an already commercial process because impact on both the product and associated regulatory filings will need to be considered. This process can be streamlined and made more efficient by establishment of a cross-functional project team to manage selection and implementation (4).
A Project Team:Figure 1 suggests groups that should be represented on a BPC implementation project team. This team defines the scope of a BPC project and conducts a cost/benefit analysis. In defining the scope, the team must determine the required scale and whether multiple manufacturing sites in the company’s network need to be considered. Next, the team determines whether a given BPC could be used in multiple processes or whether just one application will be in the project’s scope. Finally, timeline and sourcing considerations are defined. Sourcing and warehouse advantages (e.g., inventory and cost) may be realized in using the same BPC for multiple manufacturing sites and multiple applications.
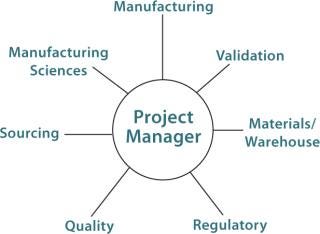
Figure 1: ()
Figure 2 is a process flow diagram for streamlining BPC implementation. End-user specifications, sourcing, and supply considerations must be taken into account with the validation package (both vendor supplied and internally generated) for a BPC when making a go–no-go decision on a particular product.
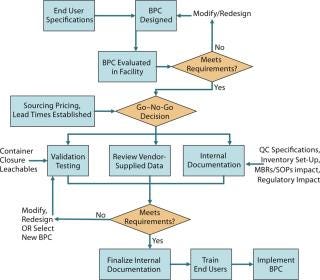
Figure 2: ()
Vendor Evaluation: Several vendors supply BPCs to the biopharmaceutical market. Once the project team is established, it should identify three or four vendor candidates. To help mitigate supply risk, it is essential to narrow those candidates to a primary and secondary vendor. Testing can be more cost-effective when evaluating both vendors’ products in parallel. During this evaluation, three sourcing requirements should be considered: lead times, customer service, and quality systems.
First, vendor lead times should be assessed. The lead time on a BPC can vary for customized and inventory items. For inventory BPCs, lead times will be greatly reduced. But most BPCs are customized. Several other factors may affect lead-times, including the choice of filter, tubing, and connections if they are integral to the bag. Presterilized BPCs are typically gamma irradiated, which can also add to lead time.
Second, a vendor’s customer service should be assessed. Response rates and vendors’ ability to answer technical questions are essential in the implementation phase of a project and will also be important throughout the lifetime of a given consumable. Failure to provide an appropriate level of customer service can delay a project and negatively affect manufacturing once a BPC has been implemented.
Third, vendor quality systems should be assessed. Quality and sourcing agreements should be made early in the process of implementation. A vendor assessment is essential for ensuring that proper quality systems are in place. It is critical that each vendor’s change control and notification systems be assessed. Future changes to a BPC component will require prompt customer notification. Vendors may conduct testing to help ensure no effects on their BPC as a result of changes made; but an end-user assessment is always needed to determine whether additional validation studies will be required. So sufficient notification is important to allow for review of validation documentation and whatever testing may be needed.
It is crucial that a BPC vendor be continuously evaluated on the lead time, customer service, and quality it provides during the lifetime of the consumable. It is possible, for example, for a vendor to provide a lead time for inventory supply that is favorable at implementation but then extend lead times after several years. Continuous evaluation ensures that each vendor continues to meet sourcing requirements. If a primary vendor no longer meets those requirements, a validated secondary vendor provides a ready alternative.
If a secondary vendor is not identified and validated, and a company wants to switch vendors, it can take considerable time to implement a new BPC. It may be possible to identify a bag, for example, from a new vendor that uses the same contact layer, but this cannot be considered a like-for-like change. An ethylene vinyl acetate (EVA) film contact layer from Vendor A can be expected to have slight differences from an EVA film contact layer from Vendor B. Vendors use proprietary formulations and have different manufacturing tolerances, so a company must account for variability between vendors. A strong information package from each one will help make a change possible, but it will not eliminate the need for end-user testing.
As has been highlighted by the Bio-Process Systems Alliance (5), both vendor and pharmaceutical manufacturer have a responsibility to provide supporting validation documentation for a given BPC. Below is a general outline of requirements for the vendor package.
The Vendor Data Package
A vendor data package review can begin once the final design of a BPC has been determined. Components that should be considered in this design include primary and secondary film, filter(s), tubing, and connections. Any or all of those components may be supplied to a BPC vendor by a third party. This review should include an assessment of physical and chemical compatibility parameters and a determination of possible gaps in supporting documentation.
That is best done through a formal technical report summarizing vendor data and comparing it with vendor specifications (5). This is a more comprehensive process than just reviewing a specification sheet. Often end users are unaware of the limitations or requirements of a consumable they’re implementing. With a formalized process, this understanding can be ensured. Below are minimal expectations for vendor data packages.
Material Properties: The material properties of each component for a BPC must be considered. End-user requirements must be reviewed against specifications for each material to ensure that it is appropriate for its intended use. End users must determine whether components need to be animal-derived component free (ADCF).
If ADCF is desirable but not possible for each component, a risk-based approach can be taken to sourcing those components for a BPC. The most desirable film would be ADCF. Tubing and connections could be evaluated through a transmissible spongiform encephalopathies (TSE) risk assessment to determine whether appropriate compliance certification can be obtained from the vendor.
Specifically for films, consider the level of scalability required for a BPC and assess the layers of film: a structural layer, a barrier layer, and a fluid-contact layer. Interaction of those layers contributes to the film’s overall performance (6).
Chemical Compatibility: The purpose of chemical compatibility testing is to determine possible chemical interactions a product solution may have on BPC components. Vendors generally test a matrix of solutions and provide a grade rating. Chemical compatibility can be evaluated by measuring changes in thickness, physical properties, color, weight, and surface quality (6). A vendor that tests a comprehensive list of solutions is desirable. Although the list will not be all-inclusive, end users should be able to compare their solutions with those on the list and determine compatibility. If there is still uncertainty after examining that list, a different vendor can be selected that has tested comparable solutions. Or solution-specific testing may be required.
Biological Compatibility: BPCs designed for use in pharmaceutical and biologics manufacturing must meet requirements for compendial tests including USP class VI, ISO, and EP testing. Several test standards demonstrate biocompatibility: USP <87>, USP <88>, USP <661>, ISO 10993, and EP 3.2.2.1. Common tests typically measure systemic toxicity, cytotoxicity, endotoxin, nonvolatile residue (NVR), residue on ignition, heavy metals, and buffering capacity. Vendor qualification should include those tests, and vendors should provide data and results to end users.
Mechanical Properties: Two typical mechanical properties are evaluated for BPC films: tensile properties and puncture resistance. Film tensile properties predict the ability of a BPC to maintain integrity, and several tests can be used to evaluate them. Tensile strength and percent elongation are the minimum tests for evaluating the tensile properties. Puncture resistance of a film predicts its durability (6). If a BPC is intended for frozen storage, its glass transition temperature should be evaluated. Evaluating the film and other components will identify the temperature at which the system becomes brittle.
Barrier Properties: Permeability is a predictor of a BPC’s ability to maintain the chemical stability, pH, and concentration of its fluid contents over time. The composition and order of the film layers are factors here (6). Standard permeability testing covers gases and vapors. Tolerance for accepting or rejecting a BPC based on permeability testing differs depending on intended storage solutions. For example, carbon dioxide permeation can alter the pH of dilute acetate buffers stored over time in certain bioprocess bags.
Shelf-Life Testing: Vendors should conduct shelf life testing for their BPC systems. Both real-time and accelerated aging testing are acceptable. This testing should be conducted following gamma irradiation at the maximum dosage level because irradiation can affect the physical and compatibility properties of BPC components and alter the system shelf life. Integrity testing is suitable to assessing an aged BPC. Vendors should evaluate their BPCs using representative components of their product lines.
Sterility Assurance: BPCs are typically presterilized for purchase. However, a vendor should conduct sterility assurance testing for its BPC system. This testing should be conducted after gamma irradiation at the minimum dosage level according to ANSI/AAMI/ISO guidelines. A vendor’s test method should include the film and a representative sample of connectors and tubing used in standard BPC systems to test a worst-case for bioburden levels.
Extractables: Plastic films contain chemical additives that assist in their manufacturing, providing strength and prolonging their shelf life. Such components may release extractables when a film is exposed to a contact solution (7). Extractables are substances that can be extracted from plastic materials using solvents and conditions that are more aggressive than actual process conditions. The type and level of extractables can vary based on contact solutions over time. Detected extractables won’t necessarily be present in a final drug product (8). Indeed, leachables are often a subset of the extractables profile for a BPC.
A complete extractables vendor package will include testing a BPC with a broad panel of solutions at exaggerated temperatures and times using a high surface/volume area ratio. Vendors can perform pH, conductivity, total organic carbon (TOC), metals analysis, nonvolatile organic compounds (NVOC), semivolatile organic compounds (SVOC), and volatile organic compounds (VOC) testing. More comprehensive studies (with identification of extractables) reduce the amount of testing required of a pharmaceutical manufacturer and allow for faster implementation of a BPC. A vendor’s extractables data forms the baseline for leachables testing that users conduct using their own process solutions and conditions. A comprehensive vendor package also can eliminate the need for end-user testing for applications that are less rigorous than the vendor’s study conditions.
Consistent extractables test methods and conditions across vendors would allow BPC end users to evaluate comparability from film to film. Elements vendors should focus on for consistency include testing contact times and temperatures, solvents used, and extractables identification levels. Although the BPSA has made important strides toward streamlining extractables testing among vendors (9), much work remains to be done in this area. Table 1 illustrates differences among vendors for extractables testing on a film used in a bioprocess bag.Table 1: Comparing extractables testing on contact films used in bioprocess bags from various vendors
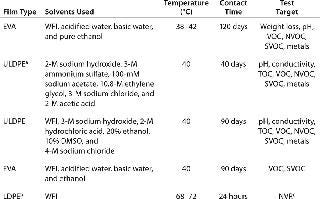
Table 1: Comparing extractables testing on contact films used in bioprocess bags from various vendors ()
End-User Testing
Depending on their applications, end users are responsible for conducting a range of tests. Early identification of which tests will be required is important. This testing can begin in parallel with evaluation of vendor data and preparation of manufacturing documentation.
Leachables: It may not be appropriate to rely completely on vendor extractables data; leachables testing may be required (10,11,12). A risk-based approach is recommended in making this decision. Leachables testing differs from extractables testing in that it uses a manufacturer’s actual process stream and conditions. Leachables are usually a subset of extractables (10) and expected to be present in a final drug product.
Table 2 compares leachables during 45-day storage of drug substance in a polyethylene bioprocess bag with the extractable profile supplied by the bag’s vendor. Table 2A compares metal leachables and extractables, showing some need to determine leachables instead of solely relying on an extractables profile. Even though the extractables were tested under extremes of solvent and temperature, the incubation time was significantly shorter than that needed for drug substance storage and transportation. By contrast, no VOCs were detected during leachables testing because of limitations in analytical sensitivity due to interference from drug substance (Table 2B). The extractables profile identified several VOCs in various solvents because of the higher-sensitivity analysis. Clearly, a combination of extractables and leachables data is needed to assess final drug toxicity effects.
Table 2A: Comparing leachables and extractables for a bioprocess bag; metal leachables detected in drug substance after storage in a bioprocess bag (mg/L) at 2–8 °C; vendor metal extractables detected in water for injection (WFI), 3-M sodium hydroxide, 4-M sodium chloride, 2-M hydrochloric acid, 20% ethanol, and 10% dimethyl sulfoxide after storage in a bioprocess bag (mg/L) at 40 °C for 90 hours
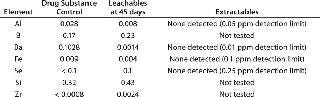
Table 2a: Comparing leachables and extractables for a bioprocess bag; metal leachables detected in drug substance after storage in a bioprocess bag (mg/L) at 2–8 °C; vendor metal extractables detected in water for injection (WFI), 3-M sodium hydroxide, 4-M sodium chloride, 2-M hydrochloric acid, 20% ethanol, and 10% dimethyl sulfoxide after storage in a bioprocess bag (mg/L) at 40 °C for 90 hours ()
Table 2B: Leachables testing for drug substance stored in a bioprocess bag for 45 days at 2–8 °C

Table 2b: Leachables testing for drug substance stored in a bioprocess bag for 45 days at 2–8 °C ()
Table 2C: Extractables testing after storage in a bioprocess bag for 90 days at 40 °C
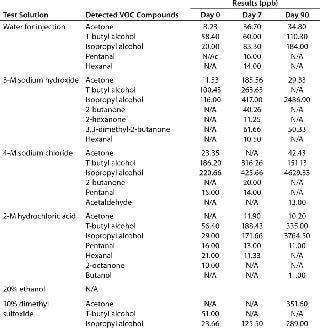
Table 2c: Extractables testing after storage in a bioprocess bag for 90 days at 40 °C ()
Manufacturers should conduct a leachables safety evaluation (13), which typically involves a toxicological assessment (14) and evaluates the potential effects of a worst-case level of leachables. Route of administration, dosing, and the ultimate number of doses will bear on this toxicological assessment. Recent literature on the potential immunostimulatory effects of certain leachables (15) is increasing emphasis on thorough evaluation of these compounds, particularly at process stages that are closest to final drug products. In some instances, a process stream may cause interference. If it is not possible to test the actual process stream, a model can be used with appropriate justification.
Compatibility Testing: A vendor’s chemical compatibility testing provides a guideline for the effects of storing basic buffers, acids, and bases in a given BPC. However, specific compatibility hold studies may be required for solutions such as media and drug substance, which may be sensitive to long-term storage. These studies should be performed using proposed storage conditions and hold times to demonstrate that a solution’s functionality is not affected by the storage in the BPC (7).
Microbial container–closure integrity testing evaluates the closure procedures an end user intends to use for a given BPC. Several tests evaluate container closure integrity. Two acceptable ones are ambient static and aerosol challenge tests.
An ambient static challenge should be conducted on all storage containers. This includes filling them with a minimum fill of trypticase soy broth (TSB) media. For situations in which multiple bag sizes use the same procedure, this challenge may be conducted on a worst-case example. Factors to consider when determining a worst case include the number of connectors and size of the bag. The container is held at room temperature for ≥14 days, being manipulated daily throughout. Manipulations include agitating the bag so its closure comes into contact with the media inside. A daily visual inspection is conducted to check for the presence of turbidity, precipitates, and pellicle and mycelia formation. Following the test period, the media is tested for growth promotion and sterility to verify whether it was capable of supporting growth if ingress occurred.
An aerosol challenge test may be appropriate for containers that hold final drug substances. This test is similar to the static challenge above except that the media filled bag is placed into an aerosolized chamber with a known concentration of Bacillis subtilis suspension for a specified duration.
The method used for container closure testing must represent a BPC’s intended use. Transportation of the system, sampling, and transfers should be considered in the experimental design, for example. And if multiple sealing methods are desired, then each one should be evaluated.
Shipping qualification studies are conducted for applications that include transporting material in BPCs to demonstrate that material integrity is not negatively affected. Two factors should be considered: temperature and shipping route.
First, a temperature study using thermocouples will demonstrate whether the necessary storage temperature is maintained during shipping. This experimental design should include a worst-case scenario for temperature and duration. The BPC should be filled to its low-fill set point because smaller volumes provide less thermal mass, thus creating a worst-case for temperature. Worst-case transport routes should be determined, so the study design can challenge worst-case time and temperature when possible (7).
A shipping route performance qualification study should be conducted to demonstrate that the shipping process has no effect on material in a BPC. Worst-case transportation routes and modes are used and include return transportation for each shipment, requiring shipping containers to experience twice the duration of a normal shipment. This challenges various handling and stop-over points along a route including handling and logistics. If possible, the actual process stream should be used for the low-fill set point, which allows the experimental design to include evaluation of the effect of worst-case agitation on the specific solution. However, a model solution can be used if small-scale agitation studies have been conducted on the drug substance, itself. Such small-scale studies should account for similar agitation that occurs over designated shipping routes, including differences among truck, train, and air transportation.
Streamlining the Process
A strong vendor package combined with appropriate end-user testing allows proper implementation of a BPC application across an entire manufacturing network. This reduces the time and cost for implementing a BPC and simplifies operator training. Using a project team and establishing vendor technical contacts early in the process can help streamline BPC implementation.
REFERENCES
1.) Novais, J, N Titchener-Hooker, and M. Hoare. 2001. Economic Comparison Between Conventional and Disposables-Based Technology for the Production of Biopharmaceuticals. Biotechnol. Bioeng. 75:143-153.
2.) Langer, E, and B. Price. 2007. Biopharmaceutical Disposables As a Disruptive Future Technology. BioPharm Int. 20:48-56.
3.) Scott, C. 2007. Single-Use Bioreactors. BioProcess Int. 5:S44-S51.
4.) Monge, M. November 2006. Successful Project Management for Implementing Single-Use Bioprocessing Systems. BioPharm Int.:S43-S51.
5.) Martin, J. 2007. Bio-Process Systems Alliance Component Quality Test Matrices: BPSA Guidelines and Standards Committee. BioProcess Int. 5.
6.) Barbaroux, M, and A. Sette. November 2006. Properties of Materials Used in Single-Use Flexible Containers: Requirements and Analysis. BioPharm Int.:S18-S29.
7.) Samavedam, R, A Goldstein, and D. Schieche. 2006. Implementation of Disposables: Validation and Other Considerations. Am. Pharmaceut. Rev. 48–51 9:46.
8.) Jenke, D. 2005. Linking Extractables and Leachables in Container/Closure Applications. PDA J. Pharmaceut. Sci. Technol. 59:265-281.
9.) Colton, R. 2008. Recommendations for Extractables/Leachables Testing: Part 2, Executing a Program. BioProcess Int. 6:44-52.
10.) Jenke, D. 2005. Strategy for Assessing the Leachables Impact of a Material Change Made in a Container/Closure System. PDA J. Pharmaceut. Sci. Technol. 59:360-380.
11.) Jenke, D, J Story, and R. Lalani. 2006. Extractables/Leachables from Plastic Tubing Used in Product Manufacturing. Int. J. Pharmaceut. 315:75-92.
12.) Yu, X, D Wood, and X. Ding. 2008. Extractables and Leachables Study Approach for Disposable Materials Used in Bioprocessing. BioPharm Int. 21:42-51.
13.) Jenke, D. 2007. An Extractables/Leachables Strategy Facilitated By Collaboration Between Drug Product Vendors and Plastic Material/System Suppliers. PDA J. Pharmaceut. Sci. Technol. 61:17-23.
14.) MacDonald, J, and R. Robertson. 2009. Toxicity Testing in the 21st Century: A View from the Pharmaceutical Industry. Toxicolog. Sci. 110:40-46.
15.) Mueller, R. 2009. Evaluation of the Immunostimulatory Potential of Stopper Extractables and Leachables By Using Dendritic Cells As Readout. J. Pharmaceut. Sci.:1-14.
You May Also Like





