Improve Process Uniformity and Cell Viability in Cryopreservation
Cell therapies and related cell-based technologies constitute an emerging, fast-growing market with a total value expected to exceed US$100 billion by 2019 (1). Cell therapy is defined as the process of introducing cells to a patient’s tissue for disease treatment. These therapies generally require cryopreservation to maintain sufficient product quality and shelf life. As a common practice, cell therapy manufacturers use controlled-rate freezers to optimize cooling profiles. The goal is to preserve final products in cryopreservation media with the highest possible cell viability while also achieving a consistent product from sample to sample and batch to batch.
Traditional controlled-rate freezers on the market are optimized for small-scale operations. As market demand increases and companies aim to control costs by increasing batch sizes, currently available controlled-rate freezers may fall short of achieving the uniform, controlled freezing processes necessary to obtain high yields and consistent products at commercial scales. Even at today’s scales, process developers face concerns that current controlled-rate freezing technology used for different clinical phases may not be scaled up to achieve sample and batch uniformity. Careful selection of cryopreservation media can alleviate some negative effects resulting from nonoptimal or nonuniform cooling profiles, but media alone cannot guarantee successful cryopreservation (2). The cell therapy industry clearly needs a controlled-rate freezing technology that can be applied at different clinical phases and easily scaled up for manufacturing without sacrificing performance (3,–5).
PRODUCT FOCUS: CELLS
PROCESS FOCUS: MANUFACTURING
WHOSHOULDREAD: PRODUCT AND PROCESS DEVELOPMENT
KEYWORDS: CELL VIABILITY, MEDIA, FREEZING/THAWING, TEMPERATURE CONTROL, DESIGN OF EXPERIMENTS
LEVEL: INTERMEDIATE
Advanced Controlled-Rate Freezing: Our company has recently developed an advanced, controlled-rate freezing (CRF) technology that enables scalable freezing process uniformity through application of a novel cooling-gas–distribution design and improved control capabilities (6). This patent-pending approach accommodates various product formats and sizes while still providing a broad range of cooling rates and final cooling temperatures.
Advanced CRF technology creates a unidirectional flow of cryogen in proximity to each sample. The cooling chamber includes a porous, cooling-gas–distribution box and an exhaust manifold for removing the cooling gas. Sample containers are placed in the cooling area between those, either directly sitting on the porous surface of the distribution box or suspended in a holding device. This design ensures that samples positioned within the cooling area are uniformly and well-controlled and fast-responsive to the temperature of the gas flowing past them.
The control system allows precise adjustment of both gas temperature and cryogen flow rate for accurate temperature manipulation in response to a desired cooling rate and/or measured temperatures in the chamber. The technology also enables robust cold-spike nucleation to controllably induce ice formation within all samples and thereby improve process uniformity and cell viability. Quick plunges in temperature are accurately and simultaneously felt by samples in the freezing chamber while heat from nucleation can be dissipated rapidly by the flowing cryogen.
The gas control techniques embodied in advanced CRF technology can be implemented at any scale. This provides continuity from development through pilot to manufacturing activities. The technology provides a novel point of control over uniformity and allows for precise cooling profiles to be developed for optimizing sample viability, as the following tests illustrate.
Materials and Methods
Cell Culture: We obtained normal human dermal fibroblast (NHDF) cells from Lonza (Walkersville, MD). Maintaining stock cultures at 37 °C in 95% air and 5% CO2 using Falcon 75-cm2 T-flasks, we grew NHDF cultures in FBM media supplemented with FGM SingleQuots (Lonza). Stock cultures were subcultured every five to six days at ∼95% confluence, and media were replenished every three days. Experiments used cell cultures between passages 2 and 10. One day before experimentation, we supplemented cultures with fresh culture media.
We obtained Jurkat A3 lymphoblasts from ATCC (#CRL-2570). Stock cultures were maintained at 37 °C in 95% air and 5% CO2 using Falcon 75-cm2 T-flasks and a complete RPMI-1640 media containing 2.5-mM glutamine (HyClone) supplemented with 10% v/v FetalClone III serum (Thermo Scientific HyClone). For cell expansion, we subcultured those stock cultures every two days, when cell density reached ∼5 × 106 cells/mL, then resuspended them in fresh T-75 flasks at a seeding density of 0.5 × 106 cells/mL. Experiments were performed using cell cultures between passages five and 12.
Cryopreservation: We used an intracellular-like CryoStor CS5 solution (BioLife Solutions) as a cryopreservation medium for the NHDF cell line. This serum-free product comes premixed with 5% v/v dimethyl sulfoxide (DMSO). For the Jurkat cell line, we prepared a solution of in-house medium with DMSO by adding 5% v/v DMSO to the complete RPMI-1640 medium containing 20% v/v FetalClone III.
We performed standard cryopreservation methods to test solutions for cryopreservation efficacy. Briefly, NHDF cells (1 × 106 cells/mL) were resuspended in 0.5 mL of the respective solutions and placed into 1.2-mL cryovials. Jurkat cells were similarly processed after cell expansion for preservation in bags by pooling cultures and resuspending to a cell density of 2 × 106 cells/mL. Cryopreservation studies involved two different advanced CRF systems. One system is designed for vials (Photo 1), and we used it to process the NHDF cell line. The other system is designed for bags, and we used it to process the Jurkat cell line.
Photo 1:

Photo 1:
To allow cell samples to equilibrate, we held samples for 10 minutes at 4 °C in the advanced CRF chamber before freezing. After the freezing program, those samples were immediately transferred to liquid nitrogen for ≤ 48 hours. We thawed them in a 37 °C water bath, then immediately resuspended them in appropriate culture media (1:10 dilution) for plating, and allowed them to recover before assessing cell viability.
Cell Viability Assessment: We assessed cell viability both qualitatively and quantitatively before freezing and after thawing the cells. For both cell lines, qualitative assessment was achieved by visualization using light microscopy. In the case of NHDF cells, quantitative assessment was accomplished using AlamarBlue (AbD Serotec). We diluted that 1:20 in Hank’s Balanced Salt Solution (Life Technologies) without phenol red (HBSS). After thawing cells and allowing them to recover in well plates for 24 hours, we removed the culture samples, mixed them with 100 µL/well of the working AlamarBlue solution, and then incubated them in the dark at 37 °C for 60 ± 1 minutes. Then we evaluated fluorescence
using a SPECTRAFluorPlus plate reader (Tecan Austria) with a 530-nm excitation and 590-nm emission filter set.
For the Jurkat cell line, quantitative assessment was accomplished using Trypan Blue staining through an automated CEDEX cell analyzer (Roche Applied Science). After thawing the cells, we resuspended a sample from the 25-mL bag in fresh medium and allowed them to recover in well plates before performing viability assessments at three and 24 hours postpreservation. From each well, we took a sample and directly measured it using the CEDEX cell counter.
Scheme for Temperature Control Study: Our temperature control studies used Praxair’s advanced CRF system (Photo 1) and a currently available commercial controlled-rate freezer with similar freezing capacity. We placed about 100 2-mL vials containing 1 mL of CryoStor CS5 solution inside the freezing chamber, inserting thermocouples into the cryopreservation medium of six vials spaced across the freezing chamber to monitor sample temperature change and heterogeneity during freezing.
Results and Discussion
Temperature Control Study: The left panel in Figure 1 shows results from the advanced CRF system temperature control study, illustrating the process temperature profiles for freezing vials in the advanced CRF chamber with cold-spike nucleation control over the period of a cryopreservation run. A solid black line indicates the temperature profile of the cold gas in response to a programmed profile for freezing NHDF cells. The typical cooling profile included five steps: (1) equilibrate at –5 °C, (2) induce ice formation with a cold spike, (3) return to –35 °C at a warming rate of 30 °C/min, (4) hold at –35 °C for 10 minutes, and (5) cool to –80 °C at a cooling rate of 2.5 °C/min. The uniformity of nucleation is apparent right after the cold gas was plunged to –80 °C, as the inset image shows. Just 20 seconds distinguish the first and last nucleation events across the monitored samples. All monitored temperature profiles were tightly aligned together through the whole freezing process. Similar results were achieved with the advanced CRF system designed for bagged products (not shown). Results from all temperature-control studies illustrate that advanced CRF technology can significantly enhance freezing-cycle homogeneity.
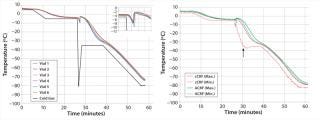
Figure 1: ()
The right panel in Figure 1 compares temperature profiles of a commercially available controlled-rate freezer (cCRF) and Praxair’s advanced CRF system using the same cooling profile described above. The figure shows the warmest (Max) and coldest (Min) sample temperatures monitored during freezing across the samples for cCRF and advanced CRF systems. We observed a larger temperature deviation for the cCRF case at critical times before and after nucleation. Maximum deviations observed for the cCRF system were ∼4 °C before nucleation and 25 °C after nucleation. By contrast, deviations for the advanced CRF system were much smaller: <1 °C before nucleation and only 5 °C afterward. Although significant temperature nonuniformity and wide sample-to-sample temperature deviations were observed with the cCRF, the advanced CRF system showed very negligible temperature deviations. That can be attributed to the novel gas-distribution and temperature-control method.
Impact of Cooling Profiles on Cell Recovery: Depending on the cell type and selection of cryopreservation media, there is an optimal cooling profile for cells to achieve highest recovery following cryopreservation (7). Advanced CRF technology provides the degree of freedom necessary for users to approach optimal cooling profiles by manipulating key variables in their cooling curves. For our study, we selected a few key variables in the cooling profile to observe how cell recovery was affected and could be tuned with the advanced CRF technology. Figure 3 shows the results from a cryo-preservation study, in which we monitored the effect of cooling variables on NHDF cell recovery following cryopreservation of cells in CryoStor CS5 solution.
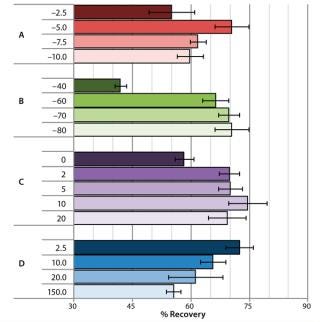
Figure 2: ()
We investigated the effect of prenucleation temperature by setting it to either –2.5 °C, –5 °C, –7.5 °C, or –10 °C while all other parameters were left unchanged in the cooling profile (Figure 2A). Optimal NHDF cell recovery was achieved when applying a prenucleation temperature of –5 °C, and cell recovery decreased with either warmer or colder prenucleation temperatures.
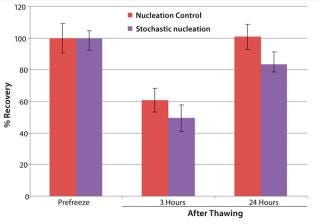
Figure 3: ()
Figure 2B shows the effect of different cold-spike temperatures on recovery of NHDF cells following cryopreservation. After the system reached prenucleation temperature, we plunged the cold gas to cold-spike temperatures of either –40 °C, –60 °C, –70 °C, or –80 °C. Optimal NHDF cell recovery was observed when we applied a cold-spike temperature of –80 °C.
Figure 2C shows the effect of variable holding times at the postnucleation temperature of –35 °C on recovery of NHDF cells following cryopreservation. A 10-minute holding time at –35 °C provided for the highest cell recovery, with a spread of ∼15% observed within the various hold times from zero to 20 minutes.
Finally, Figure 2D shows the effect of postnucleation cooling rate on recovery of NHDF cells following cryopreservation. After postnucleation holding, we cooled samples down to –80 °C at different cooling rates ranging 2.5–150 °C/min. Cell recovery decreased by 15% over the range of rates tested, with the slower postnucleation cooling rate of 2.5 °C/min providing the best results. From th
is study, we concluded that those key variables in the cooling profile did significantly affect cell viability, with recoveries ranging 42–74% by using different cooling profiles formed by manipulating key process variables.
To illustrate the advanced CRF system’s ability to work with bagged cells, we assessed Jurkat cell viability for three and 24 hours after thawing following freezing with and without nucleation control (Figure 3). In both cases, we equilibrated bags at 4 °C in the chamber before freezing. Without nucleation control, we cooled the bags after equilibration to –45 °C at a rate of 1 °C/min, then further cooled to –100 °C at a rate of 10 °C/min. Using cold-spike nucleation control, we put the bags through the following five steps after equilibration: (1) cooled to –5 °C at a rate of 1 °C/min, (2) nucleated by a cold spike, (3) warmed to 20 °C at a rate of 10 °C/min, (4) cooled to –45 °C at a rate of 1 °C/min, and (5) cooled to –100 °C at a rate of 10 °C/min.
Figure 3 shows that the cryopreservation algorithm with controlled nucleation yielded ∼10% more cell recovery at three hours after thawing and ∼20% more at 24 hours after thawing than did uncontrolled nucleation. So this cryopreservation study showed a significant impact of nucleation behavior
— and thus freezing process uniformity
— on cell recovery after thawing.
No Short-Cuts
This study examined the essential cryopreservation step for cell therapy manufacturing. Our results demonstrate the potentially significant sensitivity of cell viability to the cooling profile used during cryopreservation through controlled-rate freezing. Given that sensitivity, it is critical that a controlled-rate freezing method used in development provide sufficient uniformity and flexibility to identify the best possible cooling profile. It is equally critical that the controlled-rate freezing method used in manufacturing apply the optimum cooling profile consistently from sample to sample and batch to batch at industrially relevant scales. With its unique method of distributing and controlling the cooling gas, Praxair’s advanced CRF technology provides the necessary temperature control uniformity and flexibility. This technology offers a scalable path forward for identifying and executing optimized cooling profiles for cell therapy products.
About the Author
Author Details
Corresponding author Ying Zhou, PhD, is a development specialist at Praxair, Inc., 39 Old Ridgebury Road, Danbury, CT 06810; 1-630-320-4192, fax 1-630-320-4519; [email protected]. Zachary Fowler, PhD, is a development specialist, Alan Cheng, PhD, is a senior development professional, and Robert Sever, PhD, is senior R&D manager.
REFERENCES
1.) Jain, KK. 2010.Cell Therapy, Jain PharmaBiotech, Basel.
2.) Mathew, AJ. 2010. I’m Losing Cell Viability and Function at Different Points in My Process, and I Don’t Know Why!. BioProcess Int 8:54-47.
3.) Rowley, JA. 2011. Developing Cell Therapy Biomanufacturing Processes. SPE:S50-S55.
4.) Brandenberger, R. 2011. Cell Therapy BioProcessing. BioProcess Int 3:30-37.
5.) Buckler, RL. 2011. Opportunities in Regenerative Medicine. BioProcess Int 3:14-19.
6.) Cheng, A. 2009. Method and System for Controlled Rate Freezing of Biological Material.
7.) Karlsson, J. 1996. Fertilization and Development of Mouse Oocytes and Cryopreserved Using a Theoretically Optimized Protocol. Hum Reprod 11:1296-1305.
You May Also Like





