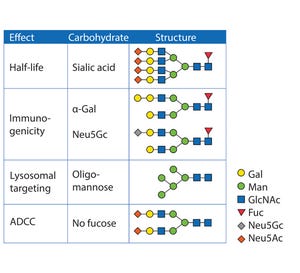
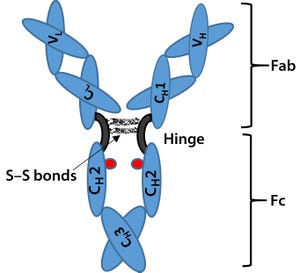
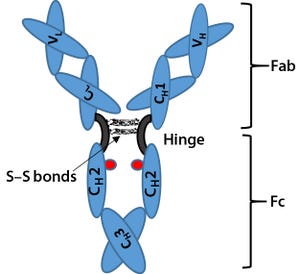
Biosimilars
Biosimilar Structural Comparison of Commercially Sourced Reference Standards by MMS Rapidly Detects Subtle but Critical Differences to Correctly
Sponsored Content
Biosimilar Structural Comparison of Commercially Sourced Reference Standards by MMS Rapidly Detects Subtle but Critical Differences to Correctly Predict Activity for Use in an ELISA ProductBiosimilar Structural Comparison of Commercially Sourced Reference Standards by MMS Rapidly Detects Subtle but Critical Differences to Correctly Predict Activity for Use in an ELISA Product