Outsourcing Biosimilars Development
May 17, 2016
VISUAL SCIENCE (WWW.VISUAL-SCIENCE.COM)
A rapid increase in the number of companies working on development and registration of biosimilars has created a significant market for contract testing and manufacturing organizations (CTOs and CMOs) providing outsourced services specific to these products. Biosimilar developers turn to contract organizations when they lack either the internal capability or capacity for conducting certain work as well as when they require additional resources to bring products to market rapidly. A wide range of contract services are available, and each particular activity offers advantages and disadvantages to biosimilar manufacturers.

Figure 1: Biosimilarity regulatory process; IND = investigational new drug; GMP/GCP/GLP = good manufacturing/clinical/laboratory practice; SA = safety assessment; BLA = biologics license application; CMO = contract manufacturing organization; CRO = contract research organization
Figure 1 depicts the regulatory process for development and licensing of a biosimilar product. The first step of a development process is obtaining and characterizing the originator product to establish a quality profile. That profile will be targeted during manufacturing biosimilar development. A biosimilar construct and cell line are developed, with extensive clone screening, manufacturing set up, and characterization of the processed drug-substance to confirm its similarity to the originator. A product formulation is then prepared and assays are developed both for quality control and to support safety and efficacy assessment.
A biosimilar product then enters the investigation new drug (IND)–enabling phase. Developers further characterize both the originator and biosimilar product to demonstrate comparability. Assays are qualified and safety assessments are conducted, if required by the appropriate regulatory authority. Discussions with the regulatory authorities are a key feature of this stage because biosimilar developers uses characterization and comparability data to minimize the requirements for animal-testing data.
Clinical trials include supporting method validation, stability studies, and manufacturing process validation required for regulatory filing. Finally, with the application granted, a product enters the postmarketing phase with testing being conducted to an approved specification, commitment stability studies, pharmacovigilance, and support of process changes.
Although development of a biosimilar product can be described as a linear process, some activities occur at multiple stages. Those include process development, characterization, and assay work. So when considering outsourcing in support of a biosimilar’s development, it is simpler for the product sponsor to consider the categories of activity involved rather than the stages supported. A biosimilar producer can then consider outsourcing a category as a whole, using a contractor’s expertise and capacity to drive a development program more rapidly. Categories to be considered include
originator procurement
analytical and functional characterization
cell line development
manufacturing development and scale up
nonclinical development
clinical development
quality control and product stability
regulatory support.
Originator Procurement
Multiple batches of the originator product are required both to establish the quality profile of a molecule and to conduct comparability studies with a biosimilar product (1). Batches must be obtained from the intended target markets and must cover the full age range from newly produced to end of shelf-life (2).
Often a biosimilar manufacturer can have difficulties obtaining a range of batches of innovator drug. Once a company decides to pursue a particular target, only recent batches might be available on the market, or a biosimilar manufacturer might lack the international reach to obtain product from all target markets. Also, a prospective biosimilar manufacturer may wish to keep its intentions confidential and thus not indicate its interest by large-scale purchase programs.
Obtaining originator batches is a relatively simple activity to outsource. Requirements can be easily stated and deliverables made clear. Active contractors have been able to identify likely targets for biosimilar manufacture and have invested in building a back catalogue of batches so that they can provide a range of ages at any time to their clients. Organizations can provide only this service or can combine it with characterization analysis or other activities.
Analytical and Functional Characterization
Analytical and functional characterization of originator and biosimilar products are critical to the success of a development program. By demonstrating a high degree of comparability (or similarity), manufacturers can reduce nonclinical and clinical testing requirements and thus decrease the costs and time to application.
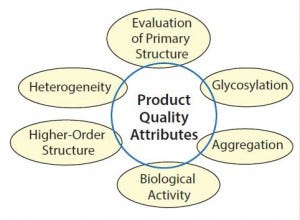
Figure 2: Main types of analytical and functional characterization
A sound understanding of a molecule, its mechanism of action (MoA), and potential impurities and degradation products during its production will assist a biosimilar applicant in discussions with regulatory authorities when considering safety and efficacy study designs. For this reason, many early entrants into the European biosimilar market (the first highly regulated market for such products) kept a large proportion of their characterization work in house. But the review and authorization process globally has matured, so the increase in numbers of batches that may need to be characterized (3), and the breadth of information required has led to a greater need for outsourcing activities.
Many aspects of an originator molecule can be characterized. Figure 2 and the related “parameters defined” sidebox provide an overview of molecular features and the techniques used to assess them.
Parameter Definitions for Figure 2 |
Heterogeneity: charge variants through sequence, modification, and degradation; key attributes include terminal sequences, deamidation, glycation, acetylation, carboxylation; methods used are cation-exchange chromatography (CEX), reversed phase–high-performance liquid chromatography (RP-HPLC), and LC–mass spectrometry (LC– MS/MS). |
Higher-Order Structure: confirmation folding and subunit combination; key attributes include secondary, tertiary, and quaternary structure; methods used include circular dichroism (CD) spectroscopy, Fourier transfer–infrared (FT-IR), nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), fluorescence, hydrogen–duterium exchange (HDX), and X-ray crystallography |
Primary Structure Determination: confirming amino acid sequence and key structures; key attributes include mass of antibodies and chains, amino acid sequence, and free and bridged cysteine; methods used include LC–electrospray ionization (ESI)-MS, matrix-assisted laser desorption/ionization (MALDI)–MS/MS, LC–MS/MS, and capillary electrophoresis-sodium dodecyl sulfate (CE–SDS). |
Glycosylation: confirming identity and distribution of glycans and sialic acids; key attributes include glycan structures, glycosylation sites, sialic acid residues; methods used include MALDI–MS/MS, LC–ESI–MS, high-performance anion-exchange chromatography with pulsed amperometeric detection (HPAEC-PAD), and CE–SDS |
Aggregation: assessment of dimers and multmers, subunit combination; key attributes include monomer combinations; methods used include size-exclusion chromatography–multiangle light scattering (SEC–MALS), field-flow fractionation (FFF), dynamic light scattering (DLS), AUC, transmission electron microscopy (TEM), and microscopy |
Biological Activity: confirmation of function, mechanism of action and potency; key attributes include receptor binding, cell uptake, signal transduction, and potency; methods used include enzyme-linked immunosorbent assay (ELISA), plasmon resonance, isothermal titration calorimetry, flow cytometry, and cell-based assays. |
Outsourcing characterization work uses greater capacity by running multiple originator and biosimilar batches as needed through a development program. And a CTO is responsible for the cost of maintaining the extensive and expensive range of instruments and full-time staff, whereas a developer may need those capabilities only over a limited time. This is particularly true for some higher-order characterization techniques, for which requirements for use are limited outside a comparability package. For biosimilar developers with less experience, the expertise of a specialized CTO in sample preparation and data interpretation can offer benefits, provided that expertise is well-communicated and available during regulatory meetings.
Demonstration of similar functional activities between originator and biosimilar products requires specialized knowledge and capability that might be unavailable within a developer’s organization. Once established, binding and potency assays lend themselves to automation, which allows high sample throughput wherever assays are conducted. But initial development of those assays requires both understanding of a product’s MoA and the suitability of techniques available. Several CTOs have invested in MoA development and validation as well as potency assays for anticipated biosimilar targets (just as procurers have obtained back catalogues of batch supplies). Thus, those CTOs can offer rapid start up for testing with a reliable method, thereby cutting development time and ensuring data consistency.
Functional and potency assays are required for quality control and stability studies and to support animal and clinical studies. These aspects of a development program require that assays operate under good manufacturing, laboratory, and clinical practice (GMP, GLP, and GCP, respectively) regulations. Some CTOs offer the benefit of transferring those methods across groups through the use of standardized platforms, thereby saving a development program from delays as alternative laboratories are established and comparability of assay performance is established.
Cell Line Development
Insertion of a product coding construct into a suitable cell line followed by selection of the most appropriate clone requires high-throughput testing and access to potential cell lines. Some biosimilar developers are turning to contract organizations to perform those services and gain access to established, well-characterized cell lines. Intellectual property concerning a cell line must be well defined for all contract agreements in which such approaches are used, and developers targeting multiple biosimilar products may wish to keep the activity in house for that reason.
The complexity of a target molecule also can play a part in a company’s decision whether to outsource cell line development work. Relatively simple molecules such as granulocyte colony-stimulating factor (G-CSF) can be produced in Escherichia coli systems, but more complex and glycosylated monoclonal antibodies (MAbs) require mammalian or specialized systems with posttranslational modifications.
Once a clone has been selected and cell line developed, master and working cell banks must be established. Banks and cells at the end of their production life are tested for purity, identity, sterility, genetic stability, and copy number. The bulk of such testing is performed only when the new banks are being established, so a biosimilar developer would need a continual stream of projects to maintain facilities and expertise in house. Use of a contractor allows immediate access to that expertise whenever required.
A master cell bank ideally would be held in at least two locations to reduce the risk of catastrophic storage failures. If a developer does not have multiple sites, then use of a contractor located close to the manufacturing facility can provide reassurance of secure, stable storage.
Manufacturing Development and Scale-Up
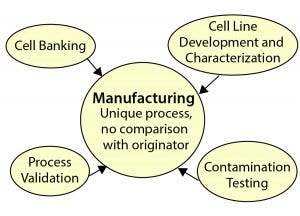
Figure 3: Biosimilar manufacturing support requirements
Figure 3 shows the manufacturing and the services that go into qualifying a process, and the “Parameters” box with it outlines those supporting requirements. Manufacturing development and scale-up present multiple opportunities for using contract organizations to support a process. Manufacture of bulk drug substances, fill and finish of a final drug product, process validation, contamination testing, and quality control can all (or in part) be outsourced.
Parameter Definitions for Figure 3 |
Cell Banking: core of a manufacturing process; local to a manufacturer or CMO; secure, stable storage at a discrete site |
Process Validation: scale-down of manufacturing process to assess ability to remove or inactivate adventitious agents; spiking with model viruses; determining log-reduction factor |
Manufacturing: same support requirements as for originators, including process development, formulation and finished product development, analytic test development and testing (quality assurance and conrol, QA/QC), scale-up, good manufacturing practice (GMP) batch production, commercial production, fill and finish, and batch release |
Cell Line Development: master and working cell banks, end of production cells; purity, identity, sterility, genetic stability, and copy number |
Contamination Testing: mouse/rat/hamster antibody production (MAP/RAP/HAP), unapparent viruses, tumorigenicity, and general safety testing; mycoplasma bacteria, endotoxin, and pyrogens (monocyte activation); process residuals (protein A, Tween, DNA, host-cell proteins). |
The choice of either internal or outsourced manufacturing is a significant decision. It depends on available capabilities, market size for a product (batch size and frequency of manufacture), and potential flexibility of a facility. Whether a manufacturer decides to establish a new plant or use an existing facility will affect the speed of bringing a product to market.
Whether production is managed internally or outsourced, process validation and contamination testing often are conducted by a service provider. Such tests involve use of positive controls for the same contaminants a process is designed to exclude and thus are not conducted within the manufacturing environment.
Nonclinical Development
Global biosimilar regulations contain provisions for animal studies to assess toxicology, immunogenicity, and pharmacokinetics/pharmacodynamics (PK/PD) (1, 4). Biosimilar manufacturers can minimize that work by discussing with regulatory agencies the closeness of quality profiles between originators and biosimilar candidates. However, some PK/PD and immunogenicity studies are likely to be required. Conducting the studies requires facilities that now are now largely confined to the CTO sector. Certainly a biosimilar producer would need to have a very significant pipeline of products to justify investment in even the smallest capability. So the choice for conducting this phase of a development program will not be whether to outsource, but rather how to select the most appropriate CRO.
To gain the maximum amount of information from preclinical studies, researchers must test collected samples using a range of techniques to assess immunogenicity, immunophenotyping, neutralizing antibody assays, and actual product levels detected through enzyme-linked immunosorbent assay (ELISA), flow cytometry, liquid chromatography mass spectrometry (LC–MS/MS), or other techniques.
A company conducting animal-trial studies must have such capabilities within easy reach. Doing so minimizes the potential for sample interaction or stability issues affecting the ability to assess samples that have been stored or shipped before testing. Data collation and interpretation through statistical analysis are key to maximizing the benefit from these studies. The experience of a CTO with biological products in general — and the class of molecule being targeted for biosimilar production in particular — can help in selection of a suitable partner. Batches of originator and biosimilar products used during those studies must be part of a characterization program. Securing sufficient material for dosing and analysis is vital.
Clinical Development
Pharmacology and immunogenicity studies are needed with comparative clinical studies to justify a biosimilar claim (1, 4). The scenario for the conduct of clinical development is very similar to that for nonclinical development: Work is outsourced to specialist providers. The choice of provider depends on geographical reach, experience with a product class, and disease indication and familiarity with the design of studies to demonstrate equivalence.
A clinical program requires a high level of bioanalytical and biomarker testing. The same methods used for nonclinical development can be applied here, with species-specific validation. So there is a strong advantage in using the same laboratories for both nonclinical and clinical phases, whether it is an internal resource or outsourced. Sample numbers are significantly higher in the clinical phase, when the distribution of test sites can introduce challenges in sample shipment and stability. Thus a proven shipping network or distribution of test sites working to the same procedures provides a significant advantage.
Quality Control and Product Stability
Here, quality control refers to the testing against specification of a bulk drug substance, finished product, and process intermediates. The scope of testing and the specifications are agreed to by a regulatory authority before clinical trials begin and revisited with the full submission. Required tests include a range of physicochemical measurements for identity, purity, potency, and microbial safety that use common techniques that are readily available in most GMP laboratories. A biosimilar developer has the choice of conducting such tests in house, contracting them to a CMO used during the product manufacture, or selecting a CTO independent of the CMO.
Keeping QC testing in-house gives a developer control of product release and builds up expertise in the purity profile of a product. Outsourcing can provide more rapid turnaround and can release internal resources to move to the next project.
Geographical location also can be a consideration in the decision of whether to outsource. If the intended market for a biosimilar includes Europe, then QC testing and product release must occur within Europe. So a laboratory in Europe (or a country with an appropriate mutual recognition agreement) would be required (5). Testing from such a laboratory can be acceptable to US authorities, but data from a US laboratory is not acceptable for European release.
Stability testing goes on throughout biosimilar product development and continues with commitment batches after product launch. Because of the standard study design, such studies require resources only at intervals, with the frequency of testing dropping as studies continue. Such programs can be awkward to schedule internally unless multiple studies are staggered. Outsourcing stability programs to CTOs prevents peaks and troughs in an internal laboratory’s workload. Stability testing uses a subset of the analytical methods used in quality control, so combining outsourcing of those two aspects minimizes the need to transfer assays to different sites.
Regulatory Support
Regulatory support from contract organizations covers all aspects of a development program, including data review from characterization, nonclinical and clinical studies, strategy development, agency meeting support, submission writing and filing through to postlaunch, and pharmacovigilance monitoring. If a biosimilar developer does not have this capability in-house, it has can use a specialist contractor or the regulatory arm of a service provider used for other aspects of a program. Using a specialist contractor offers the advantage of truly independent advice. But regulatory experts of a provider of other services will understand how those services are operated and might provide a more tailored service.
Key Roles
Through every step of a biosimilar development pathway, a product sponsor can choose to conduct the work itself or use service contractors. Contractors can be niche providers of a single aspect of a program or large, multifaceted organizations that provide multiple services. Using fewer contractors leads to greater synergy and efficiency, particularly when QC and bioanalytical methods are required. A single contractor providing multiple services offers similarity of documentation and less of an audit and management burden on a developer. If multiple services can be bundled, then developers have a stronger hand in price negotiation. However in biosimilar development, product knowledge is key, so service providers should be selected on their proven ability to work with biosimilar or originator biologic products, not on “hypothetical” capabilities.
Product developers must have clear knowledge gained from testing. Service providers can speed a development processes, but biosimilar developers must decide what knowledge to keep in house and what can be outsourced when needed.
References
1 Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. US Food and Drug Administration: Rockville, MD, April 2015.
2 EMA/CHMP/BWP/247713/2012: Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Quality Issues (Revision 1). European Medicines Agency: London, UK, May 2014.
3 Oncologic Drugs Advisory Committee Meeting, BLA 125553. US Food and Drug Administration, 7 January 2015.
4 EMEA/CHMP/BMWP/42832/2005: Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins As Active Substance: Nonclinical and Clinical Issues (Revision 1). European Medicines Agency: London, UK, May 2014.
5 Directive 2001/83/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Human use, November 2001; Official Journal L 311, 28 November 2004: 67– 128.
Niall Dinwoodie is the global coordinator of analytical testing for Charles River’s Biological Group; [email protected].
You May Also Like





