Stress-Induced Antibody Aggregates
Biomanufacturing of monoclonal antibodies (MAb) involves a number of unit operations, including cell culture in a bioreactor followed by chromatography and filtration. Purification is intended to remove impurities, such as protein aggregates, but some such operations may actually generate protein aggregation (1). Table 1 summarizes potential sources of aggregate formation during biomanufacturing processes. Aggregates are multimers of native, partially denatured, or fully denatured proteins. Their presence in biological formulations can trigger detrimental immunogenic responses upon administration (2). Moreover, aggregates can lower the efficacy and stability of a biopharmaceutical product. During biomanufacturing, therefore, it is imperative to remove aggregates to an acceptable level, which is typically <1% for MAbs.
Table 1: Potential sources of aggregation during antibody manufacturing

Table 1: Potential sources of aggregation during antibody manufacturing ()
Removing aggregates, especially soluble ones, is highly challenging because of their close physical and/or chemical similarity to the product itself (usually a monomer). Typical MAb purification processes use ion-exchange, hydrophobic-interaction, or mixed-mode chromatography for aggregate removal (4,5,6). Although such resins are effective for removing aggregates, their poor aggregate/monomer selectivity can lead to low monomer recovery when elution peaks must be “shaved” to reduce aggregate content in eluted fractions. Use of ceramic hydroxyapatite (CHT) for efficient removal of aggregates was reported in 2008 and 2009 (7, 8). But CHT chromatographic resin has its own disadvantages — namely mechanical instability, low reusability, and high cost — which to some extent can be addressed by modifying solution conditions and buffers.
PRODUCT FOCUS: ANTIBODIES
PROCESS FOCUS: MANUFACTURING, DOWN STREAM PROCESSING
WHO SHOULD READ: ANALYTICAL, PROCESS DEVELOPMENT, MANUFACTURING
KEYWORDS: MONOCLONAL ANTIBODIES, AGGREGATION, HYDROXYAPATITE, SIZE-EXCLUSION CHROMATOGRAPHY
LEVEL: INTERMEDIATE
Here, we report on our attempt to understand the nature of aggregates formed during some unit operations involved in MAb manufacturing. We evaluated different methods that mimic large-scale manufacturing unit operations for their generation of MAb aggregates by subjecting a MAb to a range of physical and chemical treatments. Our goal was to generate aggregates that are stable over time, reproducible, and comparable in “quality” to those found in industrial product streams. Generating such a standardized aggregate-containing feed that mimics “real” aggregates would help us understand and prevent aggregate formation and help enable our company to develop and evaluate novel and more efficient aggregate-removal technologies.

In addition, using such surrogates could enable testing the robustness of downstream purification (DSP) processes for cases in which unexpected increases in aggregates might occur during cell culture scale-up (3). Having a surrogate feed could enable process development scientists to test DSP unit operations early on and thus be prepared to make necessary process modifications. That could help them lower process risk and prevent delays in producing clinical and commercial material.
Materials and Methods
Materials: We used a CHO-produced MAb in our work: 95.7% monomer, 3.4% dimer, and 0.9% high–molecular-weight aggregates (HMWAs) based on measurements using size-exclusion high-performance liquid chromatography (SEC-HPLC). We purchased buffers from Sigma Aldrich of St. Louis, MO and ceramic hydroxyapatite (CHT) type II media with a mean particle size of 40 μm from Bio-Rad laboratories of Hercules, CA. And we purchased 6.6-mm i.d. Omnifit chromatography columns from Fisher Scientific of Pittsburg, PA.
Aggregate Generation: To generate aggregates to represent those found in industrial process streams, we tested four methods: temperature change, shear effects (air–liquid interface), high- and low-pH holding, and protein A chromatography followed by viral inactivation.
Temperature Change: For heating, we incubated 1 mL of MAb in a 1.8-mL Eppendorf tube in a water bath set at 50 °C for either five minutes or 18 hours. For freeze–thaw cycling, we froze 1 mL of MAb at –80 °C, then thawed it at room temperature until no solids were present. That cycle was repeated five times.
Shear Effects/Air–Liquid Interface: With multiple pump passes (using a piston pump), we pumped 40 mL of MAb at 40 mL/min. for 50 minutes to simulate 50 pump passes. We also pumped 20 mL of MAb at 5 mL/min. for 200 minutes to simulate 50 pump passes. To test mechanical agitation, we used a magnetic stir bar to agitate 20 mL of MAb in a 50-mL beaker at 600 rpm for 12 hours.
pH Holds: For a low-pH hold, we titrated 10 mL of MAb to pH 3.6 using 1.0 N HCl, then incubated that for 30 minutes before neutralizing to pH 7.4 using 2 M Tris base at pH 11.0 or 1.0 N NaOH at pH 13.0. For a high–pH hold, we titrated 10 mL of MAb to pH 10.0 using 10 N NaOH, then incubated for 10 minutes before titrating to pH 7.4 using 1.0 N HCl.
Protein A Step Followed By Viral Inactivation: We packed 2 mL of Prosep vA high-capacity protein A media from EMD Millipore of Billerica, MA into a 6.6-mm i.d. Omnifit column according to the manufacturer’s protocol. Then we loaded MAb to 15 mg/mL capacity, washed the column with phosphate-buffered saline (PBS), and eluted using 50 mM sodium acetate at pH 3.3.
Analytical Methods: We chose analytical SEC to quantify soluble aggregate content, using a Zorbax GF450 column from Agilent Technologies of Santa Clara, CA, on an Agilent HPLC system. All samples were centrifuged at 10,000 rpm in a microcentrifuge before we injected them onto the column. We also ran polyacrylamide gel electrophoresis (PAGE) under reducing (with sodium-dodecyl sulfate) and nonreducing conditions to determine the covalent or noncovalent nature of the aggregates. Before loading them on the gel, we diluted our samples to 0.5 mg/mL concentration using Laemmli buffer from Bio-Rad Laboratories of Hercules, CA.
To determine the presence of insolubl
e aggregates, we measured absorbance at 400 nm wavelength with a UV–visible spectrophotometer. Higher absorbance corresponds to turbidity caused by large particles.
CHT Experiments: All experiments were performed using an ÄKTA Explorer 100 system from GE Healthcare of Piscataway, NJ. We packed 1.5 mL of CHT resin according to the manufacturer’s protocol into a 6.6-mm i.d. column, then equilibrated it with 10 column volumes (CV) of 5 mM sodium phosphate at pH 6.5 (equilibration buffer). Then we loaded a 500-μL sample onto the column before eluting with a 20-CV gradient of equilibration buffer with 1 M NaCl. Regeneration was accomplished using 0.2 M sodium phosphate with 1 M NaCl at pH 6.5. We monitored column effluent absorbance (at 280 nm) and conductivity.
Results and Discussion
Table 2 shows our absorbance readings at 400 nm, with the percentage of soluble aggregates compared with total MAb present, and a corresponding percentage of MAb lost for samples subjected to different treatments. The antibody is very stable at high temperature: Even after an extended exposure of 18 hours, the samples showed no aggregates or titer loss. The antibody is also stable under five cycles of freezing and thawing.
Table 2: Aggregation-forming treatments
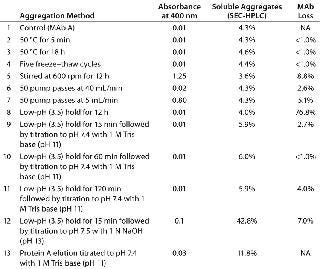
Table 2: Aggregation-forming treatments ()
Interestingly, for the methods we tested, insoluble aggregates were generated only by stirring overnight at 600 rpm and by multiple pump passes at 5 mL/min. (for 3.33 hours of total pumping time). The primary cause of that aggregation could be the presence of solid–liquid or gas–liquid interfaces rather than shear itself (9). Although both methods formed insoluble aggregates, we saw no increase in soluble aggregates over the control. So the aggregates that formed due to agitation and multiple pump passes are likely to follow a pathway in which intermediate (soluble) aggregates are highly unstable and quickly form insoluble aggregates or revert back to a monomer state. Those insoluble aggregates accounted for a 5–9% MAb loss.
We also subjected our MAb to chemical stress induced by altering the pH of its environment. Although we observed no increase in soluble or insoluble aggregates with the low-pH hold (Table 2, sample 8), considerable MAb loss (77%) occurred. That could be attributed to antibody fragmentation (based on the SEC-HPLC chromatogram in Figure 1) from exposure to the low-pH environment for an extended period. It is commonly known in the industry that one key challenge of low-pH virus inactivation is to identify an optimal hold time that allows sufficient time for virus inactivation while minimizing MAb quality issues attributable to aggregation.
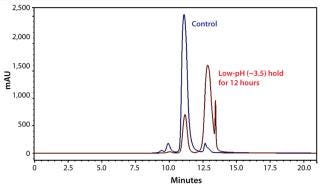
Figure 1: ()
We observed an increase in the amount of soluble aggregates only for samples 9–13. A common aspect of all those samples is a low-pH hold followed by neutralization with base. As Table 2 shows (samples 9–11), low-pH hold time does not affect aggregate generation. So the low pH in itself did not cause aggregation, but it does so when followed by titration with a base. Interestingly, titrating with 1 N NaOH at pH 13.0 induced more aggregates than did titration with 1 M Tris-base at pH 11 (Table 2, samples 9 and 12). That indicates that the type and strength of the base used for titration is critical. To better understand that observation, we titrated 10 mL of MAb to pH 10 using 10 N NaOH, held it at pH 10 for 5 min, and then titrated back to pH 7.4 using 1 N HCl. The 5-min. exposure to pH 10 generated ∼55% soluble aggregates, which indicates that high-pH exposure affects MAb stability because alkaline conditions can promote deamidation and oxidation of proteins (10).
In addition to the titrating base, solution volume might be an important variable. Table 3 shows the effect of solution volume on aggregate generation for MAb held at pH 3.5 for 15 minutes followed by neutralization with 1 N NaOH. The number of aggregates generated is inversely proportional to the solution volume (Table 3). One possible explanation for such behavior could be that for smaller operating volumes, a larger fraction of the MAb solution is exposed to localized high pH during neutralization. Another possible reason could be differences in the rate of base and acid addition, which we did not control in these experiments.
Table 3: Effect of operating volume on aggregate generation
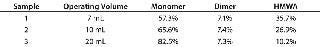
Table 3: Effect of operating volume on aggregate generation ()
Nature of Aggregates: To study the nature of these aggregates, we ran nonreducing and reducing electrophoresis gels with a MAb control and aggregate samples generated by 15 minutes of low pH (3.5–3.8) hold followed by neutralization with 1 N NaOH. The gel indicates that the pH-induced aggregates generated some covalent aggregates (Figure 2, TOP; bands above 150 kD on lane 3), which might form either because of oxidization and disulfide bond formation of free cysteine residues or due to thio–disulfide exchanges. The aggregate bands disappear in a reduced gel with addition of 5% beta-mercaptoethanol (Figure 2, BOTTOM) corroborating that the covalent bonds are disulfide in nature. Lane 5 on the nonreducing gel shows aggregate bands with lesser intensity than the monomer bands. But SEC-HPLC shows ∼68% aggregates, indicating that some of them are noncovalent and revert to monomers with sample preparation, which involves a heat-treatment step of 80 °C for two minutes. So it appears that pH treatment generates both covalent and noncovalent aggregates.

Figure 2: ()
Furthermore, we observed no significant change in SEC-HPLC profiles of the pH-change–generated aggregates stored at 4 °C over a period of two to five weeks, which indicates that the aggregates are stable over time (Figure 3). The data sets presented in Figure 3 correspond to (A) protein A elution titrated to pH 7.4, (B) low-pH hold followed by 1 M Tris titration, and (C) low-pH hold followed by 1 N NaOH titration. Data sets a and b show no major change in monomer percentage present, whereas data set c shows a 5% increase over the period studied. Interestingly, for sample set c the amount of dimers increases from 7% to 10%, whereas the HMWA reduces from 36% to 29%, indicating that the mixture has not yet reached equilibrium.
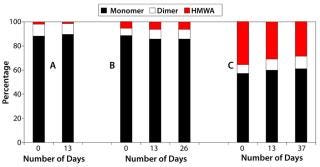
Figure 3: ()
Reproducibility and Robustness: The reproducibility with which aggregates are generated depends on a number of process variables: solution conditions, rate of addition of base, operating volume, and MAb concentration. Although the percentage of aggregates may vary, the pH-change method we chose consistently generates aggregates. Cataldo et al. have shown that the amount of aggregates can be further increased by subjecting a MAb solution to multiple cycles of high-pH hold and neutralization (11). Such pH-change–induced aggregate-generation methods have been used to produce aggregates for different MAbs at our company and elsewhere (11, 12).
Chromatographic Behavior of pH-Change–Induced Aggregates: We chromatographically tested the quality of our artificially generated aggregates using CHT type II resin. The monomer consistently elutes in the NaCl gradient, whereas aggregates elute during regeneration with 200 mM sodium phosphate (Figure 4). The ratio of peak areas in elution and regeneration closely matches with the ratio of monomer to aggregates as determined by SEC-HPLC, which indicates good separation of monomers from aggregates.
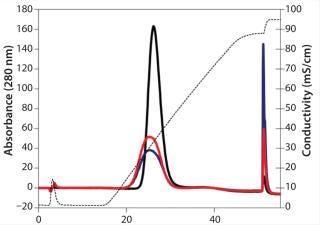
Figure 4: ()
The monomer peak for the pH-induced aggregate samples eluted earlier and was broader than that for the control (untreated sample). Such minor differences in elution profile were not observed with semipreparative strong cation-exchange or analytical weak cation-exchange chromatography (11). So the multimodal nature of protein binding to CHT might be sensitive enough to reveal such minor changes.
A Good Model
Our pH-change method can be used reproducibly to form artificial aggregates that behave similarly to native aggregates. To compare similarity, we used the criterion of chromatographic behavior because most methods used to remove aggregates are chromatographic in nature. We found that only physical stresses can create insoluble aggregates whereas chemical stresses — specifically pH shifts — can create both soluble and insoluble aggregates. The ability to create both types is critical because different methods are required to remove each type. The methods we describe here already have been applied to other antibodies, and we believe that they are general in their applicability (with small changes in the exact conditions used). Knowledge obtained from our aggregate-generation studies could be used to design new chromatographic media with aggregate selectivity.
About the Author
Author Details
Corresponding author Ajish S.R. Potty and Alex Xenopoulos are both in downstream technologies at EMD Millipore, 80 Ashby Road, Bedford, MA 01730; fax 1-781 533-5612; [email protected].
REFERENCES
1.) Cromwell, ME, E Hilario, and F. Jacobson. 2006. Protein Aggregation and Bioprocessing. AAPS J 8:E572-E579.
2.) Joubert, MK. 2012. Highly Aggregated Antibody Therapeutics Can Enhance the In Vitro Innate and Late-Stage T-Cell Immune Responses. J. Biol. Chem 287:25266-25279.
3.) Martin, JP.. High Capacity Ion Exchangers Enable a Four Gram/L Process Fit in a Two Gram/L Commercial Facility and Help Tame the Most Significant Sources of Process Variability.
4.) Yigzaw, Y. 2009. Ion Exchange Chromatography of Proteins and Clearance of Aggregates. Curr. Pharmaceut. Biotechnol 10:421-426.
5.) Lu, Y, B Williamson, and R. Gillespie. 2009. Recent Advancement in Application of Hydrophobic Interaction Chromatography for Aggregate Removal in Industrial Purification Process. Curr. Pharmaceut. Biotechnol 10:427-433.
6.) Gagnon, P. 2009. IgG Aggregate Removal By Charged-Hydrophobic Mixed Mode Chromatography. Curr. Pharmaceut. Biotechnol 10:434-439.
7.) Gagnon, P, and K. Beam. 2009. Antibody Aggregate Removal By Hydroxyapatite Chromatography. Curr. Pharmaceut. Biotechnol 10:440-446.
8.) Wensel, DL, BD Kelley, and JL. Coffman. 2008. High-Throughput Screening of Chromatographic Separations: III, Monoclonal Antibodies on Ceramic Hydroxyapatite. Biotechnol. Bioeng 100:839-854.
9.) Vázquez-Rey, M, and DA. Lang. 2011. Aggregates in Monoclonal Antibody Manufacturing Processes. Biotechnol. Bioeng 108:1494-1508.
10.) Patel, J. 2011. Stability Considerations for Biopharmaceuticals, Part 1: Overview of Protein and Peptide Degradation Pathways. BioProcess Int 10:20-31.
11.) Cataldo, W. 2012.. Comparative Characterization of Monoclonal Antibody Aggregates Formed By Two Generation Methods for Use as Model Feeds in Downstream Processing.
12.) Kuczewski, M. 2011. A Single-Use Purification Process for the Production of a Monoclonal Antibody Produced in a PER.C6 Human Cell Line. Biotechnol. J 6:56-65.
You May Also Like





