Cell-culture–related in vitro recombinant protein production is currently a $70-billion/year business. In 2007, biotech drug sales grew by 12.5%, twice as fast as standard pharmaceuticals (1). Current ongoing efforts to maximize productivity in both time and volume directly affect the scale and capital investment required for a bioreactor suite. As cells reach higher concentrations more quickly while each cell pumps out more product than ever before, the number and scale of bioreactors can be reduced. To that end, not only cell engineering, but also culture media and related chemical and physical environments are used to assist cells in reaching peak performance quickly and maintaining such a high level as long as possible.
Most culture schemes currently involve some form of nutrient supplementation after inoculation. Numerous approaches have been tried. Only by considering a number of variables can an informed decision be made to yield maximum cell productivity of a specific protein in specific cells using specific techniques. This three-part review elucidates some prominent nutrient supplementation options.
PRODUCT FOCUS: PROTEINS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: MANUFACTURING AND PROCESS DEVELOPMENT
KEYWORDS: CULTURE MEDIA, FED-BATCH, PERFUSION, MEDIA SUPPLEMENTS, AND DESIGN OF EXPERIMENTS
LEVEL: INTERMEDIATE
Photo 1:
Photo 1: ()
The basic goal of nutrient supplementation is to add depleting nutrients to cell cultures that maintain the cells in a viable and productive state as long as possible. Fed-batch and other supplementation protocols use concentrates of essential nutrients. When NaCl and other unconsumed components are omitted, more needed components can be added to a culture before osmolality increases become culture limiting. However, careful consideration is required because for some cells, phosphates and lipids (for example) can be optimized for additional productivity gains. Most attempts at fed-batch supplementation can yield a twofold or higher gain in productivity, but to consider a nutrient supplement, it is necessary to consider its relation to the type of cell culture production system used.
Overview
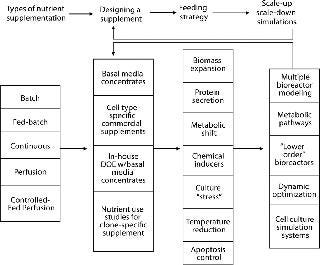
The entire process of designing, using, and verifying a nutrient supplementation strategy is dynamically interrelated and should not be thought of as a unidirectional series of events. For this discussion of component parts, however, Figure 1 suggests an overview. After choosing the type of bioreactor and supplementation process, the supplement’s chemical composition needs to be determined. A number of techniques are available, some offering significant savings in time and potential for testing a wide range of options. As provisional formulations are assessed, scale-up should be addressed. In addition to testing with larger bioreactors, scale-down models and biochemical simulations offer further help in increasing proficiency to help companies arrive at a final formulation and feeding protocol.
Figure 1: ()
Matching Supplement to Bioreactor System
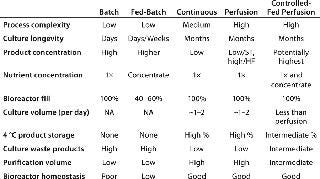
Most biomanufacturing platforms involve either a batch or a fed-batch protocol, but other production options (Photo 1) offer advantages to be considered. Table 1 shows the major bioreactor operational supplementation modes with the main characteristics of each. Optimal supplementation results require different formulations and feeding strategies because different bioreactor types work best with different kinds of supplements.Table 1: Nutrient delivery options for bioreactors
Table 1: Nutrient delivery options for bioreactors ()
In fed-batch culture, for instance, supplements should be as concentrated as possible to reduce the volume of additions because they are cumulative (volume is not withdrawn until the end of culture). So waste product accumulation may become culture-limiting. With controlled-fed perfusion culture, less concentrated supplements are used because the supplement serves not only to feed cells, but also to remove waste products for extended cultures. If a supplement is too concentrated, the low flow rate that would be acceptable for providing sufficient nutrients to the cells and that also would provide a concentrated end product would not sufficiently “flush” waste products out of the bioreactor to allow for extended culture.
Batch culture involves no supplementation. Cultures are inoculated, followed for cell viability, and harvested at the appropriate time. This is the simplest process, but culture longevity is limited by exhaustion of nutrients and build-up of waste products. As explained below, some wastes will be used toward the end of the culture.
Fed-Batch Supplementation: A typical fed-batch protocol involves adding concentrated supplemental components to a culture after inoculation. The concentrates are designed to supply needed nutrients that are consumed by the culture. Addition at the time of inoculation would increase osmolality during the growth phase, which can sometime negatively affect cell expansion. In addition, increased waste product concentrations may result from either medium component degradation due to higher initial concentrations or less efficient metabolic pathways from excess nutrient levels. Nutrient supplements usually don’t contain components that are not depleted by cells (e.g., most salts) because they would further increase osmolality, reducing the amount of supplementation possible. Although in general good results are the norm when adding supplements after inoculation, it has also been observed that comparable results may be obtained by adding a portion at inoculation.
Operational complexity is low for fed-batch processes, but the timing of specific volume additions needs to be optimized for each cell clone. Culture longevity can be increased from days for a batch up to one to two weeks with a “typical” fed-batch scheme; however, product is held for that time at incubation temperatures, so degradation must be assessed. Product concentration is higher than for batch culture, but culture waste products can affect product quality, and bioreactor homeostasis is low.
Continuous Supplementation: A culture maintained under continuous supplementation refers to continually adding 1× medium because cell concentration increases with no attempt to maintain or concentrate cells within the bioreactor. This process is more complicated than fed-batch with periodic additions, but it is operationally much less complex than perfusion. In short, 1× medium enters the bioreactor while the same volume of spent media and cells exit.
Continuous supplementation reduces the proportion of cells entering production phase at any given time because of the continual flush-out of cell populations, which results in lower titers. For labile products, continuous removal may be an advantage because product can be stored refrigerated before purification. With continuous supplementation, waste products are minimized but titers are low and purification volumes are relatively high.
Perfusion culture involves maintaining cells within a bioreactor through which 1× medium perfuses. This requires a cell concentration methodology such as spin filters, hollow fibers, acoustic membranes, or continuous centrifugation. Each of those techniques offers advantages and disadvantages. And each perfusion option will concentrate cells to different levels, which requires different supplementation strategies.
Spin filters may become plugged at some point during a process run and have to be replaced. In hollow-fiber technology, cells are maintained in an extracapillary space, with 1× medium perfusing through the capillaries. Almost tissue-dense cell masses form in the extracapillary space outside hollow fibers, yielding exceptionally high productivity per bioreactor unit volume, but nutrient and oxygen levels vary from one end of the unit to the other. Acoustic membrane technology has scale-up considerations that may preclude its use at large volumes. Nutrient delivery at very high volume exchanges has been shown to reduce cell viability presumably due to lack of self-feeding (2). Continuous centrifugation may also have scale-up issues, although multiple centrifuge units can be used.
Once a system is validated, several distinct advantages come from perfusion technologies. Cultures can be maintained for extended periods of weeks to a month or more. An equilibrium is established in which cell concentrations build and then are maintained at levels higher than other culture methods can accommodate. Viability generally holds at a relatively constant level, assuming continuous nutrient supply and waste product removal.
With hollow fibers, because cells are concentrated in the extracapillary space and depending on the molecular weight cutoff of the fibers, product may pass across the membrane and be withdrawn. It thus transfers to the perfusing 1× medium, which reduces the potential for degradation of sensitive products. One advantage for stable products is to choose a membrane cut-off limit that will keep the product in the extracapillary space. This provides for exceedingly high product concentration, which can vastly reduce the volume of supernatant to purify.
With spin filters, acoustic membranes, and continuous centrifugation operated in perfusion mode, waste products can be maintained at low levels by operating at relatively high perfusion rates. Product storage at refrigerated temperatures is possible, although purification volumes are high. Bioreactor homeostasis can be excellent in perfusion processes.
Controlled-Fed Perfusion: One additional nutrient delivery option is controlled-fed perfusion (3,4). This process combines advantages of fed-batch with those of perfusion. It involves lowering the rate of a basic 1× perfusion process by admitting smaller volumes of concentrated nutrients. The main advantage of this technique is a reduction of the large volumetric requirements of 1× media. As long as product solubility is maintained, advantages would be smaller purification volumes (higher product concentrations) with decent waste product removal and adequate nutrient levels. Success would require a relatively fine-tuned balance of reduced 1× media perfusion volumes with addition of feed supplement concentrate to maintain nutrient levels consistent with maximal cell viability and productivity. This may be the most operationally complicated form of nutrient supplementation.
Many different protein production platform options are available. Although fed-batch is the most common form of manufacture involving supplements, other options may offer specific advantages such as removal of sensitive or toxic components from cell cultures. Not only the nutrient supplement formulation, but also the feeding strategy will depend on the system used.
Identifying a Nutrient Supplement
There are several approaches to developing a nutrient supplement. Before commercial nutrient supplements were available, companies supplemented basal media such as DMEM/F12 or RPMI 1640 with available 10× concentrates of the same media or by adding powders reconstituted with 10% of the 1× volume of water. Although some improvements in monoclonal antibody productivity were noted then, it was soon recognized that supplementing a culture with all the components of a given medium adds excess NaCl and other components that quickly raise culture osmolality to detrimental levels. One improvement was to start with a basal medium and lowered osmolality, thereby allowing more supplement addition before hyperosmolality limited the culture (5). However, much better yields could be obtained with more restricted assortments of needed components.
Catalog Supplements: Several media providers now offer nutrient supplements specifically for designated cell types, such as CHO cells (e.g., Invitrogen’s CHO CD EfficientFeed kit). Biopharmaceutical companies can quickly test a range of products for possible increases in cell productivity. Information on supplement use may be included even to the point of suggesting simple mixtures and design of experiment (DOE) protocols to obtain a superior supplement for specific cell clones (6). Improvements are usually observed with these supplements.
Because cell culture media for commercial production are increasingly complex and contain higher concentrations of components than ever before, it is important to match a medium with a supplement to prevent component precipitation or chemical reactions. That may require incubating a medium with a supplement for several days at culture temperatures to rule out adverse interactions. One trend in commercial production is the use of plant hydrolysates as nutrient supplements. Although that represents an improvement over animal-based ingredients, product quality may still be an issue due to variability and possible contaminants from undefined components.
Custom Supplements: One step toward obtaining additional test supplements without involving analytical equipment is to make a single concentrate of the components in each of several basal media such as DMEM, RPMI 1640, CMRL 1066, and Medium 199, while eliminating salts and buffers. A mixtures supplementation DOE is then performed using 1× base medium followed by cell performance assays. This approach would yield a large number of supplements to test; however, it is not possible using even six or seven basal-media–derived concentrates to attain optimal concentrations for multiple components. Solubilities of different chemicals (e.g., amino acids) may lead to precipitation when different formulations are mixed together, requiring analytical work to decipher problems. Combining data from this approach with that of commercially available supplement makers will provide a relatively large number of potentially worthwhile supplement combinations for testing.
For maximizing cell productivity, it may be more advantageous to design a nutrient supplement that is specific to the clone of interest. A more analytical approach will help a company determine which components are becoming depleted in cultures and then design a replacement supplement. This requires analytical equipment for HPLC and other assays to quantify concentrations of specific components in the supernatants obtained at specific culture times (7). It is generally better to sample from multiple time points at different points in the growth curve to get a more complete picture of what supplements are needed. An optimal supplement for the growth phase may be different from one for the production phase because cell metabolism changes over the course of a culture.
Supplementing cultures with balanced, concentrated supplements at rates that prevent component depletion will keep cells in the log growth phase longer, which yields more cells for protein production. Without such nutrient balancing and replacement, specific nutrients can become depleted, initiating apoptosis. A balanced supplement contains nutrients consumed at levels reflecting their use. The resulting stoichiometrically balanced supplement (7) can be added to a culture after monitoring only one component such as glucose or glutamine. If the supplement is balanced, then all other components should remain within desired, nondepleting levels. For optimal results, a dual approach using data from both DOE and nutrient supplementation studies may provide the best clone-specific nutrient supplement (8).
Additional Supplement Considerations: Hydrolysates have been advocated as a means of improving cell productivity. They come from a number of sources and are made using enzymatic or acidic digests of animal or plant tissues such as yeast (9), soy, wheat, rice (10), and so on. Hydrolysates have been shown to increase culture longevity and productivity (11). With yeast supplementation alone, an 11.5× increase was noted in hTPO production (12) because of higher viabilities during the culture.
Although their manufacturers present extensive analytical data, plant hydrolysates remain an undefined component with potential for lot-to-lot variation due to their material sources, plant growing season characteristics, and the method and extent of bulk hydrolysis and hydrolysate purification (13). They are currently accepted for protein production processes, but as defined supplements become generally available, hydrolysate use may be deemphasized much as serum has been in the light of serum-free and chemically defined media. In addition, studies have shown comparable if not superior protein productivity using truly defined media supplements (e.g., CHO CD EfficientFeed A and B supplements from Invitrogen) compared to those containing hydrolysates (6).
Lipid supplementation has been advocated to support improved cell productivity for cholesterol-auxotrophic NS0 cells. Spens, et al. showed a doubling of cell number specifically related to cholesterol supplementation in an NS0 cell line producing human IgG1 (8). Combined with other depleting nutrients, a greater than 11-fold improvement in product yield was obtained.
Phosphate feeding has been shown to affect cell productivity (14). With NS0 cells, when phosphate was added as a supplement to a high–cell-density culture, a prolonged growth phase was observed with delayed apoptosis. This yielded a >2× cell yield and a significantly higher antibody titer. Maintenance of amino acid levels alone did not surmount the phosphate deficiency.
Removing or reducing reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide, hydroxyl radicals, or singlet oxygen has been shown to improve productivity, presumably through reduction of apoptosis. Yun et al. showed with CHO culture that a combination of glutathione and iron chelators decreased intracellular ROS levels and increased the number of viable cells (15). Addition of specific iron chelator combinations also reduced ROS and yielded improved viability. Adding several together was more effective than adding single chelators separately. Similar results were reported upon addition of ascorbic acid and glutathione as antioxidants to CHO culture by Yun et al. (16). Cell counts were higher, with fewer apoptotic cells, and the increased tPA production observed related to increased mitochondrial membrane potentials.
Sometimes a minor component not necessarily considered necessary to a nutrient supplement can actually be critical. Crowell et al. found that supplementation with amino acids and manganese modulated the glycosylation state of erythropoietin in CHO culture (17). Amino acid supplementation alone increased productivity but reduced sialylation. Manganese, a cofactor for B-1,4-galacotosyltransferase, was needed for proper sialylation — but only when added in the late culture phase.
Many Variables for Optimum Results
One of the most direct methods of gaining protein productivity is through culture supplementation with appropriate nutrients. Depending on the cell culture system used, several-fold improvements are usually possible. But product stability, purification capability, volumetric productivity, and culture system must all be analyzed to determine the optimal nutrient supplement for a given culture. Part 2 of this three-part review will discuss feeding strategies, and Part 3 will examine scale-up and scale-down strategies for rapid nutrient supplement prototyping.
REFERENCES
1.) Langer, E. 2009. Capitalizing on Novel Expression Systems. Gen. Eng. News 29:12.
2.) Dalm, M. 2004. Effect of Feed and Bleed Rate on Hybridoma Cells in an Acoustic Perfusion Bioreactor: Part I. Cell Density, Viability, and Cell-Cycle Distribution. Biotechnol. Bioeng. 88:547-557.
3.) Yang, J-D. 2000. Achievement of High Cell Density and High Antibody Productivity By a Controlled-Fed Perfusion Bioreactor Process. Biotechnol. Bioeng. 69:76-82.
4.) Feng, Q. 2006. Application of “Oxygen Uptake Rate-Amino Acids” Associated Mode in Controlled-Fed Perfusion Culture. J. Biotechnol. 122:422-430.
5.) Ryu, J, and G. Lee. 1999. Application of Hypoosmolar Medium to Fed-Batch Culture of Hybridoma Cells for Improvement of Culture Longevity. Biotechnol. Bioeng. 62:120-123.
6.) Zhao, D. 2008.Improving Protein Production in CHO CellsBioPharm Int..
7.) Wlaschin, K, and W-S. Hu. 2006. Fedbatch Culture and Dynamic Nutrient Feeding. Adv. Biochem. Engin./Biotechnol. 101:43-74.
8.) Spens, E, and L. Haggstrom. 2007. Defined Protein and Animal Component-Free NS0 Fed-Batch Culture. Biotechnol. Bioeng. 98:1183-1194.
9.) Sung, Y. 2004. Yeast Hydrolysate As a Low Cost Additive to Serum-Free Medium for the Production of Thrombopoietin in Suspension Cultures of Chinese Hamster Ovary Cells. Appl. Microbiol. Biotechnol. 63:527-536.
10.) Franek, F. 2000. Plant Protein Hydrolysates: Preparation of Defined Peptide Fractions Promoting Growth and Production in Animal Cell Cultures. Biotechnol. Prog. 16:688-692.
11.) Burteau, C. 2003. Fortification of a Protein-Free Cell Culture Medium with Plant Peptones Improves Cultivation and Productivity of an Interferon-γ–Producing CHO Cell Line. In Vitro Cell. Dev. Biol.-Animal 39:291-296.
12.) Sung, Y. 2004. Yeast Hydrolysate as a Low-Cost Additive to Serum-Free Medium for the Production of Human Thrombopoietin in Suspension Cultures of Chinese Hamster Ovary Cells. Appl. Microbiol. Biotechnol. 63:527-536.
13.) Chun, B. 2007. Usability of Size-Excluded Fractions of Soy Protein Hydrolysates for Growth and Viability of Chinese Hamster Ovary Cells in Protein-Free Suspension Culture. Bioresource Technol. 98:1000-1005.
14.) deZengotita, V. 2000. Phosphate Feeding Improves High-Cell-Concentration NS0 Myeloma Culture Performance for Monoclonal Antibody Production. Biotechnol. Bioeng. 69:566-576.
15.) Yun, Z. 2003. Combined Addition of Glutathione and Iron Chelators for Decrease of Intracellular Level of Reactive Oxygen Species and Death of Chinese Hamster Ovary Cells. J. Biosci. Bioeng. 95:124-127.
16.) Yun, Z. 2001. Effect of Antioxidants on the Apoptosis of CHO Cells and Production of Tissue Plasminogen Activator in Suspension Culture. J. Biosci. Bioeng. 91:581-585.
17.) Crowell, C. 2007. Amino Acid and Manganese Supplementation Modulates the Glycosylation State of Erythropoietin in a CHO Culture System. Biotechnol. Bioeng. 96:538-549.