Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
September 25, 2023
Date: Sep 25, 2023
Duration: 20 Min
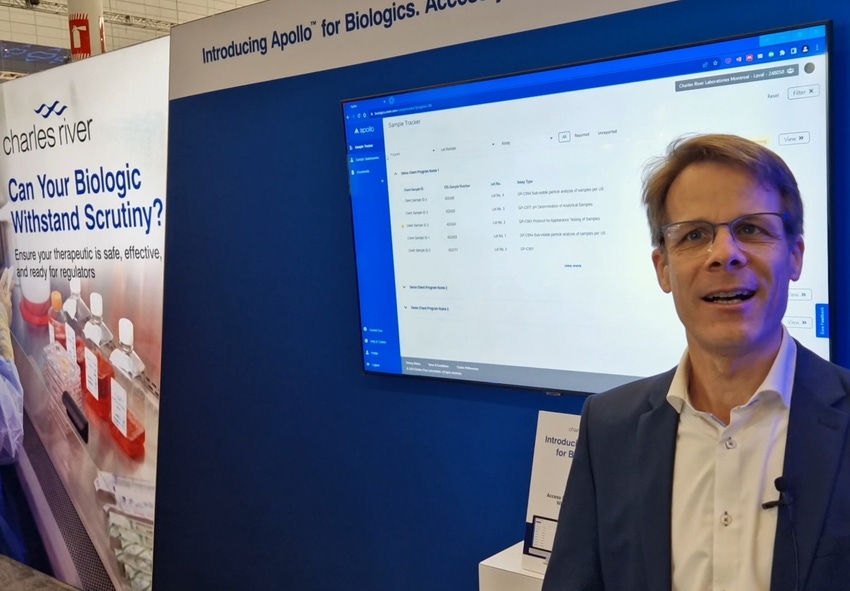
Charles River Laboratories says Apollo, it’s cloud-based secure digital platform, gives drug developers direct access to biologics sample testing data, milestones, and documents.
Among the exhibitors at BPI East (part of Biotech Week Boston), Charles River Laboratories offered demonstrations of Apollo.
The secure, cloud-based platform for drug developers aims to save time and enhance the client experience around routine biologics testing including lot-release testing – an area where velocity, visibility, and transparency is of upmost importance and could enable commercialization.
With Apollo, the customer can follow the complete sample journey from submission to reporting via: A guided sample submission wizard, adapted to the ordered work of the client and assures; A sample tracker to provide an overview of samples status with projected timelines and an extensive filter and search function; Uniform access to reports when testing has been finalized; And a secure file exchange module to exchange documents safely with colleagues.
Furthermore, the platform is connected to Charles River’s Laboratory Information Management system (LIMS) to provide nearly real-time access to the data directly out of the laboratory.
“Our goal is it to make the life of our clients easier and more efficient,” a spokesperson from the company told us. “They don’t want to spend hours with status updates and looking for their data. They want to spend time on what’s most valuable, helping patients.”
Oliver Schaefer, director of Client Experience Apollo for Biologics and Product Owner, Apollo for Biologics App, Charles River, spoke more about Apollo with BioProcess Insider at the event last week.
You May Also Like