Cleaning Validation Acceptance Limits for Biological Process Residues: Part 1 — Acceptable Exposure of Degraded Proteins Based on Reference Immunogens
May 23, 2023
Over the past decade, human therapeutic proteins (HTPs) have become far more potent, and consequently, their acceptable exposures have decreased substantially. That has led to commensurately lower acceptance limits for biological process residues. Simultaneously, host-cell and other protein concentrations have increased considerably, thereby making process equipment potentially more difficult to clean. These trends have made biopharmaceutical cleaning validation more challenging.
For example, the acceptance limits for many HTPs — based on acceptable exposures of active proteins — are on the order of 0.1 µg/cm2, which is an order of magnitude lower than the capability of most cleaning processes and analytical methods (1). As a result, acceptance limits for active protein residues often are not achievable. Thus, cleanability and cross-contamination are important regulatory considerations in biopharmaceutical manufacturing.
The degradation of HTPs during cleaning and sanitization provides a practical and effective solution to those issues. Acceptable exposures for degraded protein fragments typically are orders of magnitude greater than those of fully active proteins. Thus, the acceptance limit for degraded protein residues is commensurately greater than that for active protein residues. In addition to resolving cleanability and cross-contamination issues associated with low acceptance limits for active proteins, the higher acceptable exposures for degraded proteins provide a greater margin of safety for patients. The higher acceptable exposures also can be leveraged to simplify and streamline other aspects of cleaning validation, such as analytical method and clean-in-place (CIP) cycle development, as well as the introduction of new products (1).
In our two-part series, we describe approaches for setting safety-based acceptance limits for degraded protein residues. In this first part, we review literature on protein degradation during cleaning and sanitization and determine acceptable exposures for degraded HTPs based on clinical data for reference immunogens. In the second part, we use experimental data from in vitro immune response studies with human T cells to show that the acceptable exposure for degraded HTPs is >1 mg, which is about 100× greater than that for active HTPs.
The scope of our discussion is limited to process residues of nonconjugated HTPs. Nonetheless, the underlying principles could be useful in setting acceptance limits for other types of biological impurities, such as host-cell proteins (HCPs). The proposed methodology is intended to be broadly applicable; alternative approaches might be more appropriate for some products and situations.
Note that the acceptable exposure for a substance generally is based on both its toxicity and its immunogenicity. The former is expressed as acceptable daily exposure (ADE) or permissible daily exposure (PDE), whereas the latter is expressed as acceptable per-dose exposure (also ADE) or permissible per-dose exposure (also PDE).
Background
Protein degradation during cleaning and sanitization has important implications for many aspects of biopharmaceutical cleaning validation (2–4). The most significant implications are in setting safety-based acceptance limits for cleaning validation. That is because the acceptable exposure (and therefore the acceptance limit) for degraded protein fragments typically always is substantially higher than that of an active protein (4). As a result, the acceptance limits for degraded protein residues is always higher than that for active protein residues. Those higher limits can be leveraged to simplify and streamline cleaning validation (2, 4).
Protein degradation also has significant implications for cleaning characterization and analytical method development. For example, alkaline hydrolysis of proteins during cleaning changes the physical and chemical properties of the process soil — and thus affects its cleanability. Cleanability data for process soils are used to determine worst-case soils for cleaning validation (5–7). Further, the recovery and visual limit of detection of postcleaning residues can be significantly different from those of the process soil (8). The implications of protein degradation for cleaning characterization and analytical method development have been discussed (8). Implications for setting safety-based acceptance limits for degraded protein residues are addressed in this series.
Literature Review
Biopharmaceutical cleaning and sanitization cycles are designed to expose process soils to high pH (>13) and temperature (>120 °C) for several minutes. Under such conditions, monoclonal antibodies (MAbs) and other therapeutic proteins can degrade and denature into pharmacologically inactive fragments (Figure 1). Inactivated fragments pose an immunogenicity risk because they can lead to generation of neoepitopes. Moreover, the presence of small amounts of inactivated proteins can have an adjuvant-like effect that could modify the immunogenic potential of a therapeutic protein (9).
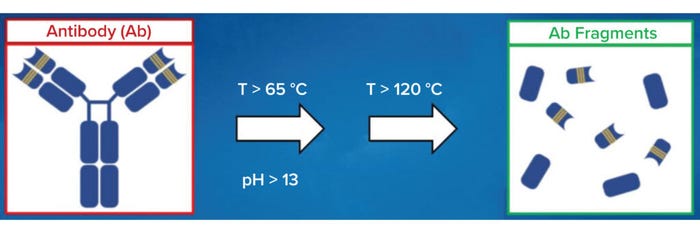
Figure 1: Antibodies and other therapeutic proteins can degrade and become inactive
during cleaning and sanitization.
Evaluation of Protein Degradation: Degradation of proteins during cleaning and sanitization can be assessed by exposing process soils to simulated operating conditions at bench scale (13). Such experiments are designed to simulate full-scale operating conditions that are least conducive, and thus worst-case, for degradation. Current regulatory expectations and science-based methodologies for bench-scale characterization of protein degradation have been discussed (10–13).
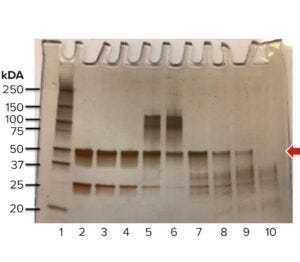
Figure 2: Evaluation of protein
fragmentation and aggregation by
sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE). The
arrow indicates the molecular weight of
the native protein (MWNP), which is
50 kDa in this case (untreated control,
Lane 2). The criterion for complete
degradation is an absence of a distinct
band at the MWNP (Lane 10).
The degree of protein degradation is evaluated by subjecting the process soil and untreated control material to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). The results are used to evaluate fragmentation and aggregation of the protein (13). Complete degradation is indicated by the absence of a distinct band at the molecular weight of the native protein (MWNP, Figure 2). Additionally, if proteins are inactivated, then the acceptance limits for postcleaning process residues can be set based on the ADE of degraded protein fragments (ADEA*). That makes the product degradation approach a science-based strategy that best reflects the phenomenological aspects of cleaning processes.
Implications for Cleaning Validation: If proteins degrade during cleaning, then the degraded protein fragments of a previously manufactured product (Product A) could be carried over into a subsequently manufactured product (Figure 3), whether that is a subsequent batch of the same product or a different product (Product B). Regardless of whether cleaning happens between batches of the same product (intracampaign cleaning, in which the subsequently manufactured product is Product A) or between batches of different products (intercampaign cleaning, in which the subsequently manufactured product is Product B), the degraded protein fragments of Product A (A*) are considered as potential contaminants. That is because the fragments are extrinsic to the manufacturing process and can be carried over into the subsequently manufactured product (4).
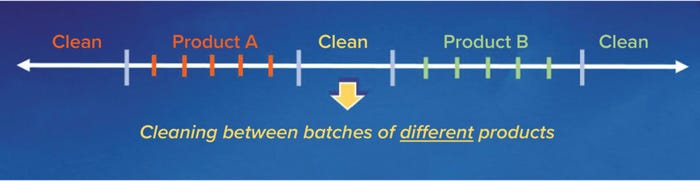
Figure 3: Typical sequence of equipment cleaning in a multiproduct facility.
Based on that rationale, and from a patient-safety perspective, the maximum acceptable carryover of A* (macA*) into the largest dose of a subsequently manufactured product is ADEA* (14): macA* = ADEA* (per dose), where ADEA* is the acceptable per-dose exposure of degraded protein fragments (A*), which are contaminants in this case (1). The asterisk implies that ADE is based on immunogenicity of the degraded protein fragments (A*). Note that ADE of the active protein (ADEA), which is based on toxicity of the active protein (A), is not relevant if the protein degrades and becomes inactive during cleaning and sanitization.
Multiplying both sides of that equation by the minimum number of doses of the subsequently manufactured product (NB,MIN) that are manufactured per production batch gives macA* × NB,MIN = ADEA* × NB,MIN (per batch). Note that doing so represents a worst case for carryover of A* and therefore a worst case for patient safety. The left-hand side of the equation thus represents the maximum acceptable carryover of A* (MACA*) into the smallest batch of subsequently manufactured product. Therefore, MACA* = ADEA* × NB,MIN (per batch). Also, NB,MIN is the smallest batch of Product B (SBB) divided by the largest dose of Product B (LDB), thus:
Equation 1
MACA* = ADEA* × (SBB ÷ LDB).
Thus, in regard to patient-safety, Equation 1 gives the maximum acceptable carryover of degraded protein into a subsequently manufactured batch of Product B. Equation 1 is used to set cleaning validation acceptance limits for process residues of degraded proteins (2, 4).
Implications for Cleaning Characterization: The physical and chemical properties of process soils — and therefore their affinity to equipment surfaces — can change significantly during cleaning and sanitization. For example, aggressive cleaning conditions (typically pH >13.5 and temperature >60 °C) are conducive to aggregation, precipitation, and gelation of proteins and peptides. The resulting drop in solubility and diffusivity of these molecules can adversely impact the cleanability and recovery of proteinaceous soils. Additionally, recoveries and visual limits of detection of postcleaning residues after exposure to cleaning chemicals can be significantly different from those of process soils before cleaning. Further, aggregates of proteins and peptides can be more immunogenic than their monomeric counterparts (15). Those factors can have implications for setting acceptance limits for cleaning validation (1, 3, 4).
Implications for Analytical Method Development: Protein degradation during cleaning and sanitization also has important implications for analytical method development. A method that is suitable for recovering and quantifying an active protein might not be as suitable for recovering and quantifying degraded fragments and aggregates of that protein (16).
For example, total organic carbon (TOC) analysis, a commonly used test method for detecting soluble proteins in cleaning-validation samples, might not be suitable for detecting insoluble aggregates of proteins and peptides. That is because TOC analysis involves oxidation of soluble organic carbon in samples, with subsequent detection of the resulting carbon dioxide. Thus, the recovery of insoluble aggregates in postcleaning residues is likely to be inadequate for accurately quantifying their concentration in swab and rinse samples.
Changes in the properties of process soils also have implications for analytical method development. For example, immunoassays — e.g., enzyme-linked immunosorbent assays (ELISAs) and enzyme immunoassays (EIAs) — can give false-negative results. They would indicate the absence of active protein if the epitopes that the assays are designed to detect have degraded during cleaning — even though the protein might remain functionally active (17).
Regulatory Expectations and Standard Practices
An important regulatory expectation for pharmaceutical cleaning validation is that the carryover of potential contaminants into drug products must be acceptable for patient safety. The ability of a cleaning process to reduce contaminants to acceptable levels is also an important regulatory expectation in preparation of newly manufactured and reusable medical devices before they are disinfected and sterilized. In either case, the criterion for patient safety generally is specified in terms of the ADEs of contaminants (2, 18–20).
Based on those regulatory expectations, an important criterion for reusable equipment in relation to the contaminant is that carryover into the largest dose of subsequently manufactured products must be less than the ADE of the contaminant (2, 4). This criterion of cross-contamination (A* → A or A* → B) generally is evaluated by collecting swab and rinse samples and testing them for A*.
Implications for Setting Acceptance Limits for Cleaning Validation
As discussed above, the degradation of HTPs during cleaning and sanitization has significant implications for setting acceptance limits for cleaning validation. That is because the ADE of a degraded HTP (ADEHTP*) is substantially greater than that of the active HTP (ADEHTP). For example, ADEHTP* is known to be >1 mg based on experimental data from in vitro immune response studies (21), whereas ADEHTP is typically <0.1 mg based on MAb clinical data. Based on clinical data for reference immunogens, the experimentally derived value of ADEHTP* is shown to be consistent with that obtained using orthogonal approaches
(4, 22).
ADE of Degraded Protein Based on Reference Immunogens: The ADE of degraded protein fragments in process residues can be set based on comparable quality (CQ) (4, 23). With the CQ approach, ADEHTP* is based on clinical data for a reference immunogen (REF): ADEHTP* = ADEREF. The immunogenicity of the reference must be comparable to or worse than that of the degraded protein fragments for predictive safety.
Predictive safety for degraded HTP fragments is evaluated in terms of the key factors that determine toxicity and immunogenicity. Because toxicity is determined based on pharmacological activity, it is not relevant to inactive protein fragments (4). Immunogenicity is determined primarily by “foreignness” and chemical complexity (15). The latter increases with molecular weight (MW); thus, larger biomolecules tend to be more immunogenic (15). The most active immunogens tend to have MWs >100 kDa (12). Protein fragments with MWs <10 kDa generally are weak immunogens (12). In fact, such small polypeptide fragments often are nonimmunogenic in vaccines and consequently need to be conjugated to large immunogenic carrier proteins or administered with adjuvants to ensure an antibody response (9).
ADE of Degraded HTP Fragments Based on CHO Protein As a Reference Immunogen: The ADE of Chinese hamster ovary (CHO) HCP has been evaluated in animal models (3). CHO HCPs serve as a good reference immunogen for inactive HTP fragments for the following reasons:
• Compared with MAbs and other HTPs, CHO proteins are more likely to elicit an immunogenic response in humans because they are of animal origin.
• Compared with intact proteins, degraded protein fragments typically are lower in MW and considered to be less immunogenic (11). The MW of HTP fragments is likely to be less than that of CHO proteins, which typically range from 10 to 100 kDa (24).
Safety-assessment studies for CHO proteins have yielded an ADE of 0.1 mg/dose (22). In those studies, cynomolgus monkeys (Macaca fascicularis) were coadministered a dose consisting of 1.0 mg of keyhole limpet hemocyanin (KLH) and 0.0, 0.1, 1.0, or 10.0 mg of CHO protein. The objective was to determine an acceptable dose level of CHO protein based on toxicity and immunogenicity (the ability of the CHO proteins to alter normal KLH antibody responses). Doses of CHO protein were administered subcutaneously once a week for four weeks, whereas the doses of KLH were administered on days 1 and 15. Samples were analyzed for cutpoint titers of anti-KLH immunoglobulins M and G (IgM, IgG) as listed in the Table 1. No adverse clinical observations, body-weight or food-consumption changes, clinical pathology or macroscopic findings, organ-weight observations, or microscopic indications were associated with this dosing regimen.
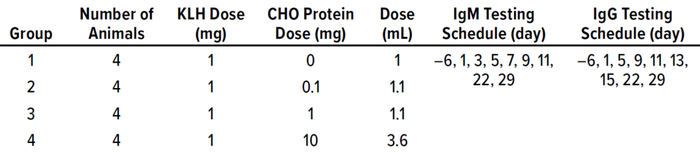
Table 1: Study parameters for determining acceptable exposure to Chinese hamster
ovary (CHO) proteins; doses are listed per animal. No adverse clinical observations were
associated with these dosing regimens. KLH = keyhole limpet hemocyanin.
Interpretation of antibody-titer data focused on peak titers (Cmax), time to peak titer (Tmax), and area under the titer–time curve (AUC). Results indicated that coadministration of CHO protein with KLH produced a dose-related decrease in Tmax for both IgM and IgG. The trend-analysis results indicated that 1 mg or 10 mg CHO protein per dose resulted in an increased AUC and Cmax for anti-KLH IgG antibody response, confirming the dose-related decrease in Tmax. However, 0.1 mg CHO protein per dose had no statistically significant effects on either AUC or Cmax. Those results indicate that 0.1 mg CHO protein per dose administered subcutaneously once a week for four weeks was well tolerated in cynomolgus monkeys and had no impact on their immune response to KLH. Thus, ADEHTP* based on CHO protein as a reference immunogen is >0.1 mg.
ADE of Degraded HTP Fragments Based on Gelatin As a Reference Immunogen: Gelatin is used as a stabilizer in many parenteral products (4). It serves as a good reference immunogen for inactive HTP fragments for the following reasons: Gelatin consists of a mixture of animal-protein fragments derived from the hydrolysis of collagen, a protein that is commonly found in connective tissues (4). It is hydrolyzed by exposing such tissues to pH and temperature extremes (25). HTPs in process residues are exposed to similar operating conditions during cleaning and sterilization. Thus, in terms of chemical composition, the mechanisms of degradation are similar for gelatin production and HTPs during cleaning.
To elicit an immune response, a molecule must be recognized as “nonself” (15). The protein fragments in gelatin are of animal origin, whereas the HTP fragments in the process residue are technically of human origin (even if they have been expressed recombinantly). Thus, the peptide sequences in HTP fragments are more likely to be recognized as “self” by human immune systems than are the peptide sequences of the protein fragments found in gelatin. Consequently, compared with the protein fragments in gelatin, the HTP fragments in process residue are less likely to elicit an immunogenic response in humans. The molecular weights of most HTP fragments are <10 kDa (4), making them weak immunogens (15). Collagen protein fragments in gelatin range from 15 kDa to 400 kDa (4), which is substantially higher than the 10-kDa threshold for weak immunogens.
Table 2 lists the amount of gelatin in some common parenteral products. The amount of gelatin in those products is at least 11,000 µg or 11 mg/dose. Thus, based on gelatin as a reference immunogen, from a predictive-safety standpoint, ADEREF is 11 mg/dose.

Table 2: Amount of gelatin found in common parenteral products formulations
(information from their package inserts).
ADE of Degraded HTP Fragments Based on the Active HTP As a Reference Immunogen: For degraded HTP fragments, the corresponding active HTP serves as a good reference immunogen for a number of reasons:
• Because the active HTP is intact, its MW would be greater than that of the fragmented HTP, making it more immunogenic than its fragments.
• Peptide sequences in HTP fragments would be comparable to those in the active HTP, so the response of human immune systems to both immunogens would be comparable.
• Dosing information for most active HTPs is readily available from dosing studies for early phase products and from package inserts for commercial products.
Based on the above rationale and the active HTP as a reference immunogen, ADEREF for active HTP is the clinical dose of the product: ADEHTP* = DoseHTP.
Example: Consider an equipment train that is used to manufacture the HTPs listed in Table 3. Determine ADEHTP* based on the corresponding active HTP as a reference immunogen and compare that with values obtained for CHO protein and gelatin as reference immunogens.
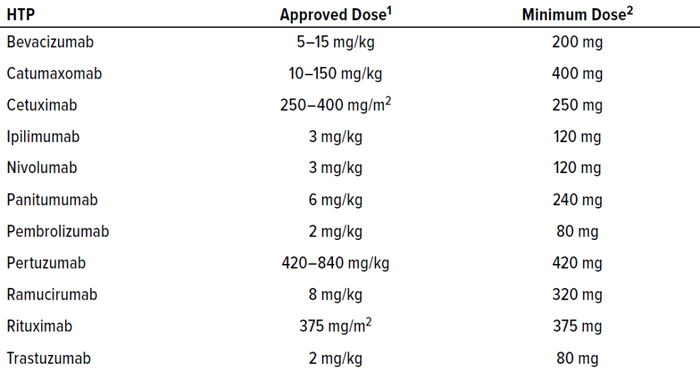
Table 3: Dosing regimens for human therapeutic proteins (HTPs) manufactured in a
shared equipment train.
1 Hendrikx JMA, et al. Fixed Dosing of Monoclonal Antibodies in Oncology. Oncologist 22(10) 2017: 1212–1221; https://doi.org/10.1634/theoncologist.2017-0167.
2 Based on surface area of 1 m2 or body weight of 40 kg (lower end of ranges for adults).
Because toxicity is not relevant for inactive HTP fragments, the ADE can be determined based solely on immunogenicity. Also, the dosing information in Table 3 indicates that ADEREF for the active HTPs is 80–420 mg, so those HTPs are nonimmunogenic at an 80-mg dose. It follows that their inactive HTP fragments also would be nonimmunogenic at the same dose.
Based on that rationale, ADEHTP* for the products listed in Table 3 is at least 80 mg. Thus, with the active HTP as a reference immunogen, ADEHTP* is 80 mg for the products manufactured in this equipment train.

Table 4: Acceptable per-dose exposure
(ADE) values for degraded HTP fragments
(ADEHTP*) based on clinical data for
reference immunogens.
1 This level of exposure was determined to be safe; however, based on the design of the study, it is not necessarily the upper limit for safe exposure.
2 For the monoclonal antibodies listed in Table 3.
Table 4 summarizes the results of the above analyses.
ADE of Degraded HTP Fragments Based on In Vitro Immune Response Studies: ADEHTP* was evaluated experimentally with in vitro immune-response studies (21). The immunogenicity of degraded HTP fragments was evaluated by exposing them to human peripheral-blood mononuclear cells (PBMCs) and then measuring the immune response. Degraded HTP fragments were prepared by exposing the drug product of a highly immunogenic HTP to worst-case cleaning and sanitization conditions. The immune response (T-cell activation) was measured with human immune-cell–based assays for cytokines (immunoregulatory proteins that are secreted by cells of the immune system).
The PBMCs were challenged with inactive HTP fragments in the presence or absence of the active HTP. The cytokine assessment was used to determine whether the inactive fragments derived from an immunogenic HTP would elicit a higher innate and adaptive phase immune response when exposed to PBMCs in both the presence and absence of the active HTP. Samples without active HTP contained 1 mg of inactive fragments, and the samples with the active HTP contained 1 mg of inactive fragments in a clinical dose of that active molecule. Supernatants collected at 20 hours (innate phase) and seven days (adaptive phase) were analyzed using Luminex immunoassays (R&D Systems).
Results showed that for all samples neither the innate- nor adaptive-phase immune responses were associated with a significant increase in secreted cytokines. Thus, the presence of 1 mg of inactivated HTP fragments in a clinical dose of that HTP induced no inflammatory response in either phase. Further, based on prior clinical data, the HTP evaluated in this study was deemed to be highly immunogenic. These results indicate that ADEHTP* is >1 mg/dose. This experimentally derived estimate of ADEHTP* is consistent with what has been obtained using the CQ approach (4, 23). Part 2 will conclude this series with details of those in vitro studies.
A Science-Based Approach
Recent advances in our understanding of protein degradation during cleaning and sanitization have led to some fundamental changes in regulatory expectations and industry practices for determining ADEs of degraded protein fragments. In this series, we discuss these developments and their implications for setting acceptance limits for biopharmaceutical cleaning validation.
An important implication of protein degradation is that the ADE of degraded HTP fragments typically is an order of magnitude greater than that of ADEHTP. For example, ADEHTP* is 11 mg based on gelatin as a reference immunogen (4) and >1 mg based on experimental data from in vitro immune response studies (21, 27). By comparison, ADEHTP typically is <0.1 mg based on MAb clinical data.
The maximum acceptable carryover of a contaminant (MACA*), and therefore the acceptance limit for that contaminant (ALA*), is proportional to its ADE (ADEA*) as calculated by Equation 1. Thus, based on our analysis, the acceptance limit for degraded protein residues (ALA*) would be an order of magnitude greater than that for active protein residues (ALA). The higher acceptance limits can be leveraged to simplify and streamline many aspects of cleaning validation, including analytical-method and CIP-cycle development, and to reduce cycle times and cleaning-related failures.
References
1 Sharnez R. Degradation of Proteins During Cleaning and Sanitization, Part 2: Implications for Setting Rational Acceptance Limits. Adv. Pharm. Valid. 6(5) 2022.
2 Sharnez R, et al. Strategies for Setting Rational MAC-Based Acceptance Limits for Cleaning Validation, Part 3: Leveraging Toxicology and Cleanability Data. J. Valid. Technol. 17(3) 2011: 24–28.
3 Sharnez R, et al. Leveraging Acceptable Exposure of Host Cell Protein to Set Acceptance Limits for Inactivated Product. J. Valid. Technol. 18(3) 2012: 38–44.
4 Sharnez R, et al. Acceptance Limits for Inactivated Product Based on Gelatin As a Reference Impurity. J. Valid. Technol. 19(1) 2013.
5 Sharnez R, et al. In Situ Monitoring of Soil Dissolution Dynamics: A Rapid and Simple Method for Determining Worst-Case Soils for Cleaning Validation. PDA J. Pharm. Sci. Tech. 58(4) 2004: 203–214.
6 Sharnez R. Leveraging Small-Scale Models to Streamline Validation. J. Valid. Technol. 14(4) 2008.
7 Sharnez R. Streamline New Product Introductions with Master Soils. J. Valid. Technol. 15(4) 2009: 24–29.
8 Sharnez R. Simplify and Streamline Recovery Studies, Part 2: Limitations and Drawbacks of TOC. Adv. Pharm. Valid. 6(3) 2022.
9 Hanly W. Overview of Adjuvants. Biologic Resources Laboratory. University of Illinois College of Medicine: Chicago, IL, 1995.
10 del Hierro G, et al. Using Bioassays in Support of Carryover Calculations in Multiproduct Facilities. J. Valid. Technol. 2021.
11 Kendrick K, Canhoto A, Kreuze M. Analysis of Degradation Properties of Biopharmaceutical Active Ingredients As Caused by Various Process Cleaning Agents and Temperature. J. Valid. Technol. 15(3) 2009: 69.
12 Rathore N, et al. Bench-Scale Characterization of Cleaning Process Design Space for Biopharmaceuticals. BioPharm Int. 22(3) 2009: 40–45; https://www.biopharminternational.com/view/bench-scale-characterization-cleaning-process-design-space-biopharmaceuticals.
13 Sharnez R, et al. Methodology for Assessing Product Inactivation During Cleaning – Part 1: Experimental Approach and Analytical Methods. J. Valid. Technol. 18(4) 2012: 42–45.
14 Sharnez R. A First-Principles Approach to Cleaning Validation, Part 1: Simplify Validation with a Simple Mass Balance. Adv. Pharm. Valid. 1(1) 2022.
15 Murphy K. Janeway’s Immunobiology (Eighth Edition). Garland Science: New York, NY, 2012: 719–720.
16 Sharnez R. Degradation of Proteins During Cleaning and Sanitization, Part 1: Implications for Cleaning Characterization and Analytical Method Development. Adv. Pharm. Valid. 6(4) 2022: https://www.researchgate.net/publication/360917367_Advances_in_Cleaning_Validation.
17 GUI-0028. Cleaning Validation Guide. Health Canada: Ottawa, Ontario, Canada, 29 June 2021; https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/validation/cleaning-validation-guidelines-guide-0028/document.html.
18 EMA/CHMP/CVMP/SWP/169430/2012. Guideline on Setting Health-Based Exposure Limits for Use in Risk Identification in the Manufacture of Different Medicinal Products in Shared Facilities. European Medicines Agency: Amsterdam, The Netherlands, 20 November 2014; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-setting-health-based-exposure-limits-use-risk-identification-manufacture-different_en.pdf.
19 Section 10: Cleaning Validation. EU GMP Annex 15: Qualification and Validation. European Commission: Brussels, Belgium, 2015; https://www.gmp-compliance.org/files/guidemgr/2015-10_annex15.pdf.
20 Baseline Guides, Volume 7. Risk-Based Manufacture of Pharmaceutical Products (Second Edition). ISPE: North Bethesda, MD, July, 2017.
21 Jawa V, et al. Cleaning Validation Acceptance Limits for Inactivated Therapeutic Proteins Based on Human Immune Cell-Based In Vitro Analysis. unpublished manuscript.
22 Sharnez R, et al. Strategies for Setting Rational MAC-Based Acceptance Limits for Cleaning Validation, Part 2: Application to Rinse Samples. J. Valid. Technol. 17(2) 2011: 43–46.
23 Sharnez R, To A. Acceptance Limits for Inactivated Product, Part 1: The Comparable Quality Approach. J. Valid. Technol. 17(4) 2011: 32–36.
24 McNally G. Process Validation: A Lifecycle Approach. US Food and Drug Administration: Rockville, MD, 6 May 2011; https://variation.com/wp-content/uploads/guidance/Presentation-Process-Validation-A-Lifecycle-Approach-Grace-McNally-2011.pdf.
25 Sharnez R, VanTrieste M. Chapter 6: Quality-By-Design for Cleaning Validation. Cleaning and Cleaning Validation, Volume 1. Davis Healthcare International: Scottsdale, AZ, 2009; Parenteral Drug Association: Bethesda, MD, 2009.
26 Sharnez R. Strategies for Setting Rational MAC-Based Acceptance Limits for Cleaning Validation, Part 1: Reassessing the Carryover Criterion. J. Valid. Technol. 16(1) 2010: 71–74.
27 Sharnez R, et al. Cleaning Validation Acceptance Limits for Biological Process Residues, Part 2: Acceptable Exposure of Degraded Proteins Based on In Vitro Immune Response Studies. BioProcess Int. 2023, accepted.
Acknolwledgments
We’re grateful to the following colleagues for their contributions: Brenna Overman
and Chris Cowley (AGC Biologics), Michael Hendershot (Eli Lilly), Ryan Takeya (Seagen), and Vibha Jawa (Bristol-Myers Squibb).
Further Reading
CBER/CDER/CVM. Guidance for Industry: Process Validation — General Principles and Practices. US Food and Drug Administration: Rockville, MD, January 2011: https://www.fda.gov/files/drugs/published/Process-Validation–General-Principles-and-Practices.pdf.
ICH Q7: Good Manufacturing Practice for Active Pharmaceutical Ingredients. US Fed. Reg. 66(186) 2000: 49028–49029; https://database.ich.org/sites/default/files/Q7%20Guideline.pdf.
Inspection Guide: Validation of Cleaning Practices. US Food and Drug Administration: Rockville, MD, 1993; https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/validation-cleaning-processes-793.
Sharnez R. Adopting the Product Lifecycle Approach. BioPharm Int. 28(1) 2015: https://www.biopharminternational.com/view/adopting-product-lifecycle-approach.
Warner RC. The Alkaline Hydrolysis of Egg Albumin. J. Biolog. Chem. 142, 1942: 741–756.
Corresponding author Rizwan Sharnez, PhD, is president of Cleaning Validation Solutions, 3506 Vale View Lane, Mead, CO 80542; 1-970-535-2130; [email protected]. Andrew Brewer is a principal engineer at Genentech (Roche); Darragh Halpin is a validation manager at Amgen; Ina Hammond is a validation manager at Agilent Technologies; Jeffery Felker is a deputy director at Sanofi; Kathleen Bellorado-Kendrick is a validation manager at Pfizer; Laura Goldberg is a validation director at Agilent Technologies; Loleta Chung is an associate director at Boehringer-Ingelheim; Maria Delgado-Rentas is an associate validation manager at Regeneron Pharmaceuticals; Mary Gibson is an associate vice president at Agilent Technologies; Michael Parks is a director at Pfizer; Paul Hefner is a senior director at Novavax; Rajyalakshmi (Raji) Vathyam is a validation manager at Takeda Pharmaceutical Company; Sushil Abraham is a vice president at Alexion; Timothy Riehlman is a staff analytical scientist at Regeneron Pharmaceuticals; Xiaona Wen is a senior scientist at Merck & Co. Inc.; and Yunjuan (Jane) Wang is a principal scientist at AstraZeneca.
You May Also Like





