Membrane-Based Clarification of Polysaccharide Vaccines
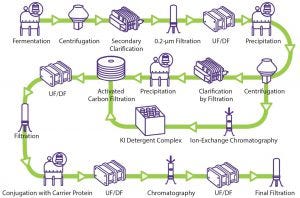
Figure 1: General polysaccharide conjugate vaccine process (UF/DF = ultrafiltration/diafiltration)
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1).
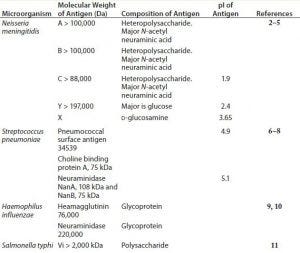
Table 1: General polysaccharide antigen properties
Polysaccharides are relatively complex carbohydrates made up of many monosaccharides joined together by glycosidic bonds. Bacterial polysaccharides represent a diverse range of macromolecules that include peptidoglycans, lipopolysaccharides, capsules, and exopolysaccharides — compounds whose functions range from structural cell-wall components (e.g., peptidoglycan) to important virulence factors (e.g., Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae). Most polysaccharides produced by bacteria are of high molecular weight with acidic isoelectric points (pI). Table 1 shows general properties of polysaccharide antigens.
Polysaccharide antigens consist of repeating epitopes that are not processed by antigen-presenting cells (APCs). These antigens interact directly with B cells, which can induce antibody synthesis in the absence of T cells (known as T-independent antigens). T cells can influence the antibody response to certain polysaccharides, such as the capsular polysaccharide of S. pneumoniae type 3. T-independent responses fail to induce significant and sustained amounts of antibody in children below the age of 18 months (1, 2). In 1929, Avery and Goebel demonstrated that the poor immunogenicity of purified S. pneumoniae type 3 polysaccharide in rabbits could be enhanced by conjugation of the polysaccharide to a protein carrier. That led to the foundation for the development of conjugated polysaccharide vaccines (1).
Modern polysaccharide vaccines are generally conjugated to nontoxic, nonreactogenic carrier proteins or tetanus toxoid (a 150-kD protein from the Gram-positive anaerobic bacteria Clostridium tetani). Alternatively, CRM 197 (a single point-mutated 68-kD protein purified from Cornybacterium diptheriae or as recombinant protein expressed in Eshcherichia coli or Pseudomonas) has been the carrier protein of choice in several vaccines. The selection of a carrier protein can be driven by a number of factors, including availability, price, and chemical characteristics such as stability at certain pHs and adjuvant effect (2).
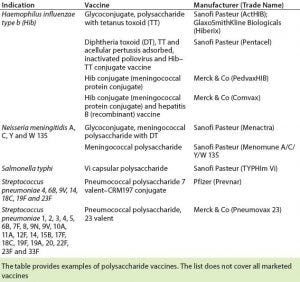
Table 2: Licensed carbohydrate-based vaccines (13)
Several polysaccharide-based vaccines are in current development pipelines. Carbohydrate-based vaccines in development are being derived from Streptococcus, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysenteriae, Shigella flexneri, Shigella sonnei, Vibrio cholera, Leishmania species, and others (3). Table 2 shows a selection of carbohydrate vaccines that have been licensed.
There are several other carrier proteins used as conjugation partners for polysaccharide vaccines. Pneumococcal surface protein A (PspA) is one such carrier protein (3). Another is protein D (PD), a 42-kDa surface lipoprotein found in all Haemophilus influenzae, including nontypeable (NT) H. influenzae used as an antigenically active carrier protein in an 11-valent pneumococcal conjugate investigational vaccine (4). Diphtheria toxoid (DT), tetanus toxoid (TT), and CRM197 also have been used as protein carriers in licensed vaccines (5).
Vaccines for N. meningitidis are based on outer membrane vesicles between 20 and 200 nm. Outer membrane vesicles (OMVs) are released spontaneously during growth by multiple Gram-negative bacteria. OMVs have lipopolysaccharides, phospholipids, proteins, RNA/DNA, and peptidoglycan, and they are produced by bacterial fermentation. Centrifugation has been implemented for recovery. Tangential-flow filtration (TFF) cassettes (10–100 kDa) also have been used to separate OMV from small soluble components (6). TFF provides a range of surface antigens that are in native conformation and possess natural properties such as immunogenicity, self-adjuvation, and uptake by immune cells. Such characteristics make them attractive for applications as vaccines against pathogenic bacteria. An OMV-containing meningococcal vaccine (Bexsero from Novartis) was recently approved by US and EU regulatory agencies, and research on its application continues.
Despite growing interest, no established template exists for a vaccine purification platform because of the complexity and diversity of vaccines (7). The manufacturing process of vaccines can be divided into three segments: upstream processing, downstream processing (purification), and formulation (fill–finish operation). Figure 1 illustrates a generic conjugated polysaccharide vaccine production process.
Clarification is an essential operation in the production of biological products because it directly affects yield, product consistency, and reproducibility. The goals of clarification include high yield, product consistency, and reproducibility. Primary clarification removes the majority of large particles, whole cells, and cell debris. This step can be performed by TFF–microfiltration (TFF–MF), centrifugation, or (in some cases) depth filtration. Secondary clarification is used to remove colloids, lipids, DNA–RNA, residual cells, and other particles not removed in a primary clarification process. Secondary filtration typically includes a series of filters to remove progressively smaller particles (8).

Table 3: Combination of technologies used for clarification of vaccines
Traditionally, a combination of technologies has been used for the clarification of conjugated polysaccharide vaccines, as summarized in Table 3. Because centrifugation can handle high solid loads, it has been the preferred method. Generally, initial cell mass is in the range of 7–30 OD at 590 nm or can reach 5 g/L dry cell weight (9). Centrifugation can concentrate cell mass to about 40% of the initial volume (8). Clarifying filtration can be performed by normal-flow filtration (NFF, also known as dead-end filtration) or TFF (also known as cross-flow filtration). Depth filters contain positively charged material and filter aid that enhance retention of cell debris, colloids, and unwanted negatively charged components (8, 10). Membrane filters retain particles by size exclusion, but they have limited dirt-holding capacity and are more suitable for a secondary clarification step.
Scalability is not a concern with depth filters or membrane filters. TFF is used mostly for primary clarification (microfiltration) with successful cut-offs in the range of 0.1–0.65 µm (preferably with open channel). Linear scalability and reusability of TFF devices significantly reduces costs of consumables in the clarification step (11).
Improper optimization of clarification steps can affect the performance of subsequent downstream unit operations in terms of capacity of filters or life of membranes and resins. With a need for well-characterized vaccines, simplified processes, as well as increased purity, new filtration technologies can handle clarification challenges for more process flexibility, possibility of single-use, and reduced investment costs.
Clarification of Polysaccharide Vaccines
Considerations for Polysaccharide Vaccines Clarification: After fermentation of polysaccharide vaccines, the next step is harvest clarification. For high-packed cell volumes, direct filtration through normal-flow filters is not economically feasible because of low throughput. In most cases, centrifugation is common practice for separation of cell mass. TFF–MF also could be used (12–14). Filtrate from the TFF–MF step can be further clarified using NFF depth filtration train, followed by bioburden reduction by filtration. In some cases, homogenization can be implemented to enhance performance of clarification (15).
Buffer conditions and pH can affect polysaccharide clarification.Hadidi et al. demonstrated the effect of ionic strength and pH on the hydrodynamic radius of the free polysaccharide and conjugated polysaccharide, which then had an impact on retention time during size-exclusion chromatography (SEC) (16). The study also showed that retention time of the pneumococcal polysaccharides increases in SEC columns with increasing ionic strength and decreasing pH because of compaction of the polysaccharides associated with a reduction in intramolecular electrostatic interactions.
Strategy for Polysaccharide Vaccines Clarification
Primary Clarification Step: Centrifugation is the preferred technology for separating high cell mass from fermentation broth. Depending on scale, continuous or batch centrifugation could be used. General centrifugation conditions are 14,000–15,000g for 45–60 minutes (17, 18).
TFF at microfiltration (MF) range can be used as an alternative to centrifugation. The molecular weight of polysaccharides that are large and complex in structure typically ranges from about 500 kDa to over 1,000 kDa. MF membranes (e.g., 0.22 µm, 0.45 µm, and 0.65 µm) are preferred to ensure successful recovery of polysaccharide molecules in permeate because of their large open pore size.
Secondary Clarification Step: The clarity/turbidity of cell-free fermentation broth depends on the specific bacteria, lysis type, individual serotype, and technology used for primary clarification. Turbidity of postcentrifuge centrate could range from about 50 NTU to 1,300 NTU. After primary clarification steps, NFF with depth media (e.g., B1HC, C0HC, F0HC, or X0HC grades of Millistak Pod disposable depth filters from MilliporeSigma) could be used to achieve turbidity <5–10 NTU, suitable feed levels for further purification steps.
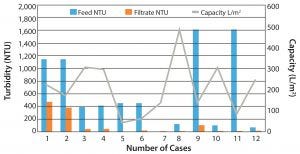
Figure 2: General depth filter performance for clarification of harvest
Volumetric throughputs on the depth filter can range from about 30 L/m2 to 500 L/m2. Depth filter clarified product fluid could be filtered through a subsequent 0.45-µm bioburden-reduction–grade or 0.22-µm sterilizing-grade membrane. In general, performance of depth filters varies among different polysaccharide vaccines, and values and performance might vary from case to case. Feed can range between 100 NTU and 10,000 NTU. Depth filters in general have shown capacities of 50–300 L/m2. In the postprimary clarification, the filtrate turbidity can drop down to <50 NTU. Figure 2 shows performance of several depth filters.
Case Studies
Clarification of Postcentrifuge Centrate of S. pneumoniae Fermentation Broth: The traditional method of clarification is centrifugation, followed by time-consuming, multiple-step depth filtration. Some researchers have made efforts in developing single-step, filtration-based clarification methods for polysaccharide vaccines. In one study, charged depth filters were implemented for clarification of S. pneumoniae serotype 9V fermentation broth, resulting in 10- to 13-fold reduction of turbidity from 130 NTU (data not published). Such one-step clarification operated at flow rate of 50–60 L/m2/h (LMH) resulted in completion of clarification of a 2,000-L batch in about three to four hours. This translated to a volumetric throughput of about 180–200 L/m2. Similar filtration performance also was observed with S. pneumoniae serotype 20F, 12F, and 17F.
As a result of this study, the method of centrifugation for clarification of pneumococcal harvest was changed to depth filtration. The ideal way to judge the effect of a change in clarification method is to observe performance of downstream filtration. If filtrate quality improves, downstream filtration performance ideally would improve in terms of capacity (volumetric processing) and can lower differential pressure due to slower plugging.
Clarification of Postcentrifuge Centrate of H. influenzae Fermentation Broth: Centrifugation is generally the preferred choice for primary clarification. But in 2008, Takagi evaluated a two-way approach (19). First, H. influenza B fermentation broth was loaded onto depth filters. Filtration was performed using a train of two charged depth filters. Reduction of feed turbidity was observed from about OD 5 to less than OD 0.1 at 650 nm. Tests were performed at 10–30 LMH, and 300 L were processed in about 3–4 hours with capacities of about 20–30 L/m2.
The other approach was to subject the harvest to microfiltration TFF (using two pump-based, permeatecontrolled operation) as an alternative, reusable option to depth filters (20). After this TFF processes the feed, OD of the filtrate decreased from 5 to <0.1. The flux was 10 LMH, with loading of about 30–40 L/m2. Area requirement for processing 300 L of volume was 10 m2 in three hours (unpublished data).
If possible, single-step TFF is a good alternative to centrifugation and secondary clarification. Scaling-up or scaling down centrifuge is not easy. Depending on specific conditions, TFF cassettes can be used for multiple batches.
Clarification of Salmonella typhi Vi Harvest: Inactivated Salmonella typhi bacteria can be clarified using a two-step process. As an option for primary clarification, a TFF device (0.45-µm cassette) was used to concentrate bacteria cells 7–10-fold and then diafiltered 10 times against 1M NaCl. During cell concentration, some Vi polysaccharides passed through the membrane into the permeate. A significant quantity of Vi polysaccharides is generally retained because of the ionic interaction between the negatively charged Vi polysaccharides and other positively charged components in the broth. A high salt concentration can be used to weaken those interactions — neutralizing the strong negative charge on the Vi polysaccharides (at pH ∼7.0), facilitating passage of Vi polysaccharides through membrane pores, and resulting in increased recovery of Vi polysaccharides (12, 13). Typical volumetric loading for such operation is 45–55 L/m2 and the processing can take three to five hours.
As a secondary clarification option, 30-kDa TFF can be used on the permeate pool from first TFF step for concentration (10–15 fold) and diafiltration (8–10 times) against water for injection (WFI). During this step, Vi polysaccharides will remain in the retentate, and low–molecular-weight impurities and excess water would pass through the membrane into the permeate. Diafiltration against water was required to remove excess salt to make negatively charged Vi polysaccharides readily accessible to the positively charged Cetavlon for subsequent precipitation step (12, 13). Typical volumetric loading for such operation is 45–55 L/m2, and the processing can take 3–5 hours, resulting in about 100% recovery.
TFF coupled with changes in solubility or ionic interactions facilitate clarification procedures and help reduce contaminant load and size-dependent impurities. This results in high recovery of polysaccharides.
Clarification of Postcentrifuge Centrate of Fermentation Broth By Addition of a Flocculating Agent: Adding flocculating agent to a bioreactor before centrifugation can improve depth-filtration–based clarification. A study conducted in our laboratory evaluated multiple depth filters to select an appropriate secondary clarifying filter. The study found that implementation of a Millistak+ C0HC filter (MilliporeSigma) alone as a secondary clarification filter on centrate reduced turbidity by ∼90% (from feed NTU 100, to 10 NTU). Filtration using a Millistak+ C0HC filter was performed at ∼500 LMH, resulting in a capacity >400 L/m2 (unpublished data). With a Millistak+ C0HC filter, a minimal ∆P was observed, suggesting that the centrifuge is removing larger particles, and the Millistak+C0HC filter (with a tighter nominal pore size) is probably removing smaller particles.
Those results indicate that with proper screening of depth filters, a filtration train can be reduced while still achieving a desired throughput and reduction in turbidity. In turn, that may result in smaller footprint and ease of operation. In our study, only one step depth filtration achieved the desired clarification of centrate.
A number of next-generation filtration products are being introduced to enable better, easier, and more robust clarification applications. Kang et al. observed that Clarisolve filters (from MilliporeSigma) can be implemented for better clarification of flocculated monoclonal antibody harvest (14). Those filters are more open, with pore sizes 20–60 µm in range.
In a study on pneumococcal vaccine, Clarisolve 60HS (polypropylene) filters (from MilliporeSigma) were evaluated on a harvest pretreated with celite and cetyltrimethylammonium bromide (CTAB). This study resulted in loading of 50 L/m2 and found that performance of Clarisolve filters as primary clarifying filters do not depend on cell viability and cell count. Implementation of Clarisolve filters for pretreated polysaccharide vaccine harvest can omit centrifugation and secondary clarification steps, resulting in a smaller footprint and easier operation.
Next-Generation Clarification Methods
Clarification of polysaccharide vaccines presents several challenges. Typically, filtration processes and filtration trains vary case by case. Because of high cell mass, centrifugation or microfiltration devices typically are preferred for primary clarification. Of late, charged depth filters have shown promise for primary clarification, and membrane filters are proving to be better options for secondary clarification.
Templates for purification of polysaccharide vaccines are being implemented or adopted, and clarification schemes by filtration are delivering high degrees of success due to robustness, ease of scalability, and process economics. Improvement in clarification steps have resulted in higher final yields and purity in vaccine processes. As new clarification products, tools, and solutions are being made available, vaccine developers and producers will continue to be better prepared for efficient and effective clarification processes.
References
1 Goldblatt D. Conjugate Vaccines. Clin. Exp. Immunol. 119, 2000: 1–3. doi: 10.1046/j.1365-2249.2000.01109.x.
2 An SJ, et al. Physicochemical Properties of Salmonella typhi Vi Polysaccharide: Diphtheria Toxoid Conjugate Vaccines Affect Immunogenicity. Vaccine 29, 2011: 7618–7623. doi: 10.1016/j.vaccine.2011.08.019.
3 Astronomo RD, Burton DR. Carbohydrate Vaccines: Developing Sweet Solutions to Sticky Situations? Nat. Rev. Drug Discov. 9(4) 2010: 308–324. doi: 10.1038/nrd3012. PMID 20357803.
4 Forsgren A, et al. Protein D of Haemophilus influenzae: A Protective Nontypeable H. influenzae Antigen and a Carrier for Pneumococcal Conjugate Vaccines. Clin. Infectious Diseases 46, 2008: 726–731. doi: 10.1086/527396.
5 Tontini M, et al. Comparison of CRM197, Diphtheria Toxoid and Tetanus Toxoid as Protein Carriers for Meningococcal Glycoconjugate Vaccines. Vaccine 31(42) 2013: 4827–4833. doi: 10.1016/j.vaccine.2013.07.078.PMID 23965218.
6 van der Pol L, et al. Outer Membrane Vesicles as Platform Vaccine Technology. Biotechnol. J. 10(11) 2015: 1689–1706. doi: 10.1002/biot.201400395.
7 Ball P, et al. 21st Century Vaccine Manufacturing: Examining the Potential of Rapid Analytical Methodologies and Worldwide Supply Chains. BioProcess Int. 7(4) 2009: 18–28.
8 Yavorsky et al. The Clarification of Bioreactor Cell Cultures for Biopharmaceuticals. Pharma. Technol. 2003; 62–76.
9 Fu J, et al. Recent Advances in the Large-Scale Fermentation of Neisseria meningitidis Group B for the Production of an Outer Membrane Protein Complex. Biotechnol. 13(2) 1995: 170–174. PMID 9634759.
10 van Reis R, Zydney A. Membrane Separations in Biotechnology. Curr. Op. Biotechnol. 12(2) 2001: 208–211. doi: 10.1016/S0958-1669(00)00201-9.
11 Besnard L, et al. Clarification of Vaccines: An Overview of Filter-Based Technology Trends and Best Practices. Biotechnol. Adv. 34(1) 2016: 1–13. doi: 10.1016/j.biotechadv.2015.11.005.
12 Kothari S, et al. A Novel Method for Purification of Vi Capsular Polysaccharide Produced By Salmonella enterica Subspecies Enterica Serovar Typhi. Vaccine 31(42) 2013: 4714–4719. doi: 10.1016/j.vaccine.2013.08.037; PMID 23994374.
13 Kothari S, et al. Development of an Efficient and Scalable Method for Processing and Purification of Vi Capsular Polysaccharide. Proc. Vaccinol. 2, 2010: 78–81. doi: 10.1016/j.provac.2010.03.014.
14 Gonçalves VMM, et al. Purification of Capsular Polysaccharide from Streptococcus pneumoniae Serotype 23F By a Procedure Suitable for Scale‐Up. Biotechnol. Appl. Biochem. 37(3) 2003: 283–287. doi: 10.1042/BA20020075.
15 Andre BR, Champluvier BPS, inventors; Glaxosmithkline Biologicals SA, assignee. Method for Producing Virus from Cell Culture Involving Homogenization. WO2010128100A1, 11 November 210.
16 Hadidi M, et al. Effect of Solution Conditions on Characteristics and Size-Exclusion Chromatography of Pneumococcal Polysaccharides and Conjugate Vaccines. Carbohydrate Polymer 152, 2016: 12–18. doi: 10.1016/j.carbpol.2016.06.095.
17 Pisal SS, Reddy CS, Reddy PS, inventors; Serum Institute of India Ltd, assignee. Production of High Yields of Bacterial Vaccines. WO 2014080423A2. 30 May 2014.
18 Peddireddy SR, et al., inventors; Serum Institute of India Ltd, assignee. Bacterial Capsular Polysaccharide Yield Enhancement By Addition of Defoaming Agents. EP2952586A1. 9 December 2015.
19 Takagi M, et al. Purification of Capsular Polysaccharide Produced By Haemophilus influenza Type b Through a Simple, Efficient, and Suitable Method for Scale-Up. J. Ind. Microbiol. Biotechnol. 35(11) 2008: 1217–1222. doi: 10.1007/s10295-008-0428-4.
20 Raghunath B, et al. Best Practices for Optimization and Scale-Up of Microfiltration TFF Processes. Bioprocess J. 11(1) 2012: 30–40; http://dx.doi.org/10.12665/J111.Raghunath.
21 Liu TY, et al. Studies on the Meningococcal Polysaccharides, 1: Composition and Chemical Properties of the Group A Polysaccharide. J. Biol. Chem. 246(9) 1971: 2849–2858.
22 Liu TY, et al. Studies on the Meningococcal Polysaccharides, 2: Composition and Chemical Properties of the Group B and C Polysaccharide. J. Biol. Chem. 246(15) 1971: 4703–4712.
23 Apicella MA, Robinson JA. Physicochemical Properties of Neisseria meningitidis Group C and Y Polysaccharide Antigens. Infect Immun. 2(4) 1970: 392–397.
24 Apicella MA, Robinson JA. Physicochemical Properties of Neisseria meningitidis Group X Polysaccharide Antigen. Infect. Immun. 6(5) 1972: 773–778.
25 Jedrzejas MJ. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 65(2) 2001: 187–207. doi: 10.1128/MMBR.65.2.187–207.2001.
26 Briles DE, et al. Pneumococcal Diversity: Considerations for New Vaccine Strategies with Emphasis on Pneumococcal Surface Protein A (PspA). Clin. Microbiol. Rev. 11(4) 1998: 645–657.
27 Worthington Biochemical Corp. Neuraminidase; www.worthington-biochem.com/NEUP/default.html.
28 Gürtler L. Virology of Human Influenza. Influenza Report 2006. Kamps BS, et al., Eds. Flying Publisher: Paris, France, 2006: 87–91.
29 Shtyrya YA, et al. Influenza Virus Neuraminidase: Structure and Function. Acta Naturae 1(2) 2009: 26–32. PMID22649600.
30 Complete List of Vaccines Licensed for Immunization and Distribution in the US; www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm.
31 Perciani CT, et al. Conjugation of Polysaccharide 6B from Streptococcus pneumoniae with Pneumococcal Surface Protein A: PspA Conformation and Its Effect on the Immune Response. Clin. Vaccine Immunol. 20(6) 2013: 858–866. doi: 10.1128/CVI.00754-12.
32 Robinson A, et al. Meningitis Vaccine Manufacturing: Fermentation Harvest Procedures Affect Purification. BioPharm. Int. 24, 2011: s21–s26.
33 Kalbfuss B, et al. Harvesting and Concentration of Human in Influenza A Virus Produced in Serum-Free Mammalian Cell Culture for the Production of Vaccines. Biotechnol. Bioeng. 97(1) 2007: 73–85. doi: 10.1002/bit.21139. PMID 16921531.
34 Macha C, et al. Purification of Streptococcus pneumonie Capsular Polysaccharides Using Aluminium Phosphate and Ethanol. Int. J. Pharmacy Pharm. Sci. 6(2) 2014: 385–387.
35 Hamidi A, Haag D, Beurret MF, Bilt D, inventors. Nederlands Vaccin Instituut, assignee. Process for Producing a Capsular Polysaccharide for Use in Conjugate Vaccines. US patent 2007/0065460 A1. 22 March 2007.
36 Hamidi A, et al. Process Development of a New Haemophilus influenzae Type B Conjugate Vaccine and the Use of Mathematical Modelling to Identify Process Optimization Possibilities. Biotechnol. Prog. 32(3) 2016: 568–580. doi: 10.1002/btpr.2235.
37 Kang Y, et al. Development of a Novel and Efficient Cell Culture Flocculation Process Using a Stimulus Responsive Polymer to Streamline Antibody Purification Processes. Biotechnol. Bioeng. 110(11) 2013: 2928–2937. doi: 10.1002/bit.24969.
Nikhil Shaligram is manger of process development at Merck Life Science Pvt Ltd. (Mumbai, India). Sudeep Kothari is senior research scientist at Vaccine Process Development, Science Division, International Vaccine Institute (Seoul, Korea). Keunhoe Koo is senior process development scientist and Cheon-Ik Park is manager of biomanufacturing science and technology at Merck Ltd. (Seoul, Korea) Elizabeth Goodrich is head of applications engineering at MilliporeSigma Corporation (Billerica, MA). Corresponding author Priyabrata Pattnaik is director and head of biologics operations at Merck Pte. Ltd., 3 International Business Park, #02-01 Nordic European Centre, Singapore 609927; 65-6890-0610; fax 65-6890-6776; [email protected].
You May Also Like





